非系结的正式分子间十六氢-二烷基-阿尔德反应:硼炔酸酯与2-(1,3-丁二炔基)吡啶
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们发现,2-二炔基吡啶和一个末端为bpin的单炔或双炔会交叉反应生成苯中间体。这些活性中间体被各种原位捕获剂捕获,得到三组分反应的产物。各种控制反应、底物修饰、结合研究和DFT分析表明,少量的非共价路易斯酸碱配合物是双炔和亲二酚结合产生苯的活性物质。当使用双炔时,只形成一个同分异构体;这种选择性是由在dft计算的二元中间体中看到的几何畸变合理化的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
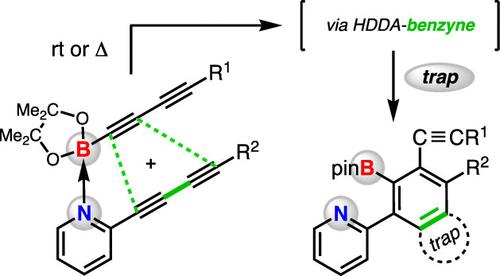
An Untethered and Formal Intermolecular Hexadehydro-Diels–Alder Reaction: Alkynylboronates with 2-(1,3-Butadiynyl)pyridines
We show that 2-diynylpyridine and a Bpin-terminated monoyne or diyne will cross-react to form benzyne intermediates. These reactive intermediates are captured by various in situ trapping agents to give products of three-component reactions. Various control reactions, substrate modification, binding studies, and DFT analysis suggest that a small amount of a noncovalent Lewis acid–base complex is the active species within which the diyne and diynophile engage to produce the benzyne. Only a single isomeric benzyne is formed when a Bpin-diyne is used; this selectivity is rationalized by the geometric distortion seen in the DFT-computed diradical intermediate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


