通过中断Polonovski策略获得含nhc催化的偶氮苯甲酸反应的β-酮氨基酸衍生物
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
介绍了一种由醛类和亚胺代物合成β-酮类氨基酸衍生物的通用而实用的方法。在中断的Polonovski策略下,原位形成的亚胺被催化生成的Breslow中间体在偶氮苯并酯反应中捕获。目前的策略已扩展到氟苯尼考的正式合成和万古霉素的中间产物。合成的β-酮氨基酸衍生物的功能化突出了当前方法的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
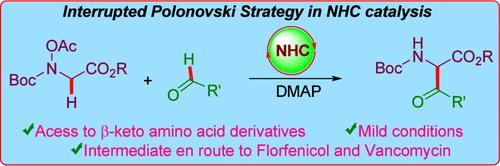
Access to β-Keto Amino Acid Derivatives via Interrupted Polonovski Strategy Involving NHC-Catalyzed Aza Benzoin Reaction
A general and practical route to the synthesis of β-keto amino acid derivatives from aldehydes and bench stable imine surrogates is presented. Following the interrupted Polonovski strategy, the imine formed in situ was trapped by the catalytically generated Breslow intermediate in an aza benzoin reaction. The present strategy has been extended to the formal synthesis of florfenicol and an intermediate en route to vancomycin. The functionalization of the synthesized β-keto amino acid derivatives highlights the practical applicability of the current methodology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


