电解液对锡金属电极上CO2电化学还原反应的影响
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
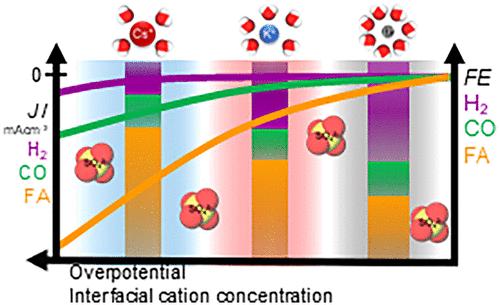
了解控制电化学CO2还原反应(CO2RR)的电解质因素是为实际应用选择最佳电解质条件的基础。虽然对贵金属的研究较多,但对电解液对锡催化剂CO2RR的影响研究较少。在这里,我们研究了电解质对Sn金属电极的影响,研究了电解质浓度、阳离子特性和阴离子特性的影响,以及它如何影响CO2RR活性和选择性。在温和酸性条件下,阳离子对甲酸和一氧化碳的活性随阳离子浓度和大小的增加而增加。相反,产氢不受阴极电位、电解质浓度和阳离子大小的强烈影响。此外,我们比较了在固定阳离子浓度的K2SO4 (pH 4)和KHCO3 (pH 7)中CO2RR的性能,结果表明,在SHE尺度上,对HCOOH和CO的反应速率受阴离子同质性的影响最小,而受溶液中阳离子的影响,我们将其归因于反应受到阳离子耦合电子转移步骤的限制,而不是质子耦合电子转移步骤的限制。我们认为HCOOH通过吸附氢化物生成*OCHO中间体,而CO通过电子转移步骤生成*CO2δ−。阳离子通过稳定带负电荷的中间体来促进这两个过程,而HCOOH对CO形成的促进程度的差异可能源于阳离子对*H的影响比*CO2δ−更强。此外,高浓度(1.0 mol L-1)的HCO3 -的存在被证明可以显著提高高过电位(-1.0 V vs RHE)下H2的生成,因为碳酸氢盐离子作为质子供体,优于水还原。这些发现强调了电解质工程对增强甲酸合成的重要性,为优化锡电催化剂上的CO2RR工艺提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrolyte Effects on Electrochemical CO2 Reduction Reaction at Sn Metallic Electrode
Understanding the electrolyte factors governing the electrochemical CO2 reduction reaction (CO2RR) is fundamental for selecting the optimized electrolyte conditions for practical applications. While noble metals are frequently studied, the electrolyte effects on the CO2RR on Sn catalysts are not well explored. Here, we studied the electrolyte effect on Sn metallic electrodes, investigating the impact of electrolyte concentration, cation identity, and anion properties, and how it shapes the CO2RR activity and selectivity. The activity for formic acid and carbon monoxide increases with the cation concentration and size at mild acid conditions. In contrast, hydrogen production is not strongly influenced by the cathodic potential, electrolyte concentration, and cation size. Furthermore, we have compared the CO2RR performance at a constant cation concentration in K2SO4 (pH 4) and KHCO3 (pH 7), where we show that the reaction rate toward HCOOH and CO are minimally impacted by the anion identity on the SHE scale, while being affected by the cations in solution, which we attribute to the reaction being limited by cation-coupled electron transfer steps rather than by a proton-coupled electron transfer step. We propose that the HCOOH forms via adsorbed hydrides leading to *OCHO intermediate, while CO forms through an electron transfer step, producing *CO2δ−. Cations facilitate both processes by stabilizing the negatively charged intermediates, and the difference in the extent of the promotion of HCOOH over CO formation would stem from the stronger cation effects on *H compared with *CO2δ− species. Additionally, the presence of HCO3– at high concentrations (1.0 mol L–1) is shown to significantly enhance the production of H2 at high overpotentials (>-1.0 V vs RHE) due to bicarbonate ions acting as protons donors, outcompeting water reduction. These findings underscore the significance of electrolyte engineering for enhanced formic acid synthesis, offering valuable insights for optimizing the CO2RR processes on Sn electrocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


