RuCo合金中的d-d轨道偶联促进了还原胺化反应中席夫碱的氨解
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
还原胺法选择性生产伯胺的研究因其广泛的应用而受到越来越多的关注,但高效的还原胺法仍然是一个挑战。本文提出了一种新的策略,通过d-d轨道偶联策略,在RuCo合金中实现羰基化合物与NH3的高效还原胺化。所设计的RuCo合金系列催化剂不仅在各种基质的转化中表现出优异的性能,而且在不同载体上具有普遍的适用性。RuCo合金的关键作用是其独特的能力,有效地催化希夫碱的氨解,这是还原性胺化反应的速率决定步骤。理论研究表明,d - d轨道耦合策略打破了金属(Co或Ru)原子的5个d轨道等效贡献,d轨道主要通过RuCo(001)表面Ru的dz2和dx2-y2轨道以及Co的dz2轨道贡献,显著推动了席夫碱氨解过程中NH3的均解离解。在较低能级下,RuCo(001)表面生成稳定的Co-H共价键和RuCo- nh2半离子键和半共价键。这项工作不仅实现了高效的还原胺化,而且提供了对非均相催化的d-d轨道耦合策略的原子轨道水平的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
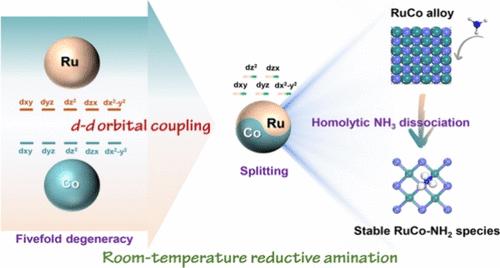
d–d Orbital Coupling in RuCo Alloy Enhances the Ammonolysis of a Schiff Base in Reductive Amination
The selective production of primary amines through reductive amination has received increasing attention due to their extensive applications, while efficient reductive amination is still challenging. Herein, a novel strategy was developed to realize the efficient reductive amination of carbonyl compounds with NH3 via d–d orbital coupling strategies in RuCo alloys. The designed series of RuCo alloy catalysts not only exhibited excellent performance in conversion of various substrates but also demonstrated universal applicability across different supports. The key role of RuCo alloy is its unique ability to effectively catalyze the ammonolysis of a Schiff base, the rate-determining step in reductive amination. Theoretical studies reveal that the d–d orbital coupling strategies break the five d orbital equivalent contributions of metal (Co or Ru) atoms, and the d orbitals primarily contribute through the dz2 and dx2-y2 orbitals of Ru and the dz2 orbital of Co on the RuCo(001) surface, which significantly driving the homolytic NH3 dissociation during the ammonolysis of the Schiff base. Furthermore, stable Co–H covalent bond species and RuCo-NH2 half-ionic and half-covalent bond species on the RuCo(001) surface were generated with lower energy levels. This work not only achieves efficient reductive amination but also provides an atomic orbital-level understanding of the d–d orbital coupling strategies for heterogeneous catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


