铜催化烯基硫铵盐的二氟甲基化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种新颖而直接的方案,促进了烯基磺酸盐的转化,从而直接合成e -二氟甲基化烯烃。该方法的成功依赖于铜催化剂和Vicic-Mikami试剂(DMPU)2Zn(CF2H)2的使用。这些温和的方案提供了选择性合成芳香族或脂肪族二氟甲基化烯烃的优点。此外,我们的方法延伸到烯基磺酸盐的全氟烷基化。值得注意的是,这种方法有利于大规模合成,并有望用于各种应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
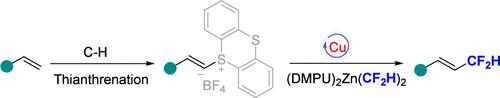
Copper-Catalyzed Difluoromethylation of Alkenyl Thianthrenium Salts
We have developed a novel and straightforward protocol that facilitates the transformation of alkenylsulfonium salts leading to the direct synthesis of E-difluoromethylated alkenes. The success of this method relies on the use of copper catalysis and Vicic–Mikami reagent (DMPU)2Zn(CF2H)2. These mild protocols offer the advantage of selectively synthesizing either aromatic or aliphatic difluoromethylated alkenes. Furthermore, our methodology extends to the perfluoroalkylation of alkenylsulfonium salts. Notably, this approach is conducive to large-scale synthesis and holds promise for diverse applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


