binap加速铜介导的芳烃晚期三氟甲基化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
使用Ruppert-Prakash试剂原位生成的CuCF3在可见光下对芳基噻吩盐进行位点选择性和后期C-H三氟甲基化(包括五氟甲基化),发现rac-BINAP的催化量显著加速,使反应在非常温和的条件下在15分钟内完成,而无需使用任何额外的光氧化还原催化剂。该工艺具有底物范围广、效率高、区域选择性好等特点,有望加快氟化药物化合物的发现。本文章由计算机程序翻译,如有差异,请以英文原文为准。
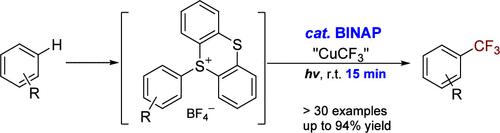
BINAP-Accelerated Copper-Mediated Photochemical Late-Stage Trifluoromethylation of Arenes
Site-selective and late-stage C–H trifluoromethylation (including pentafluoroethylation) of arylthianthrenium salts using in situ generated CuCF3 from Ruppert–Prakash reagent under visible light was found to be significantly accelerated by a catalytic amount of rac-BINAP, allowing the reaction to complete within 15 min under very mild conditions without using any additional photoredox catalysts. The process showed a broad substrate scope, high efficiency, and excellent regioselectivity, which holds the promise to accelerate the discovery of fluorinated medicinal compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


