在肝纤维化中,通过nr2f2调控的HMGB1炎症信号级联,麻菜碱A可减轻纤维化和炎症。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
本研究探讨了马钱子碱A (BA)的肝保护功能及其减轻肝纤维化的潜在机制。用TGF-β处理肝星状细胞(HSCs)和小鼠原代肝细胞,随后暴露于BA。为了评估BA对NR2F2- hmgb1信号级联的影响,这些细胞被靶向NR2F2的siRNA载体转染。NR2F2和HMGB1启动子之间的相互作用是通过双荧光素酶实验来阐明的。在体内,用硫代乙酰胺(TAA)诱导C57BL/6小鼠肝损伤,然后给药BA。研究发现,BA可调节细胞外基质(ECM)形成、上皮-间质转化(EMT)和炎症介质水平,同时降低活化hsc中NR2F2和HMGB1的表达。此外,BA减轻肝细胞的焦亡,减少炎症反应。hsc或肝细胞中NR2F2的缺失阻碍了BA对该途径的抑制作用。结果表明NR2F2直接与HMGB1启动子结合。BA治疗可降低血清ALT和AST水平,减轻肝组织损伤,降低ECM和中性粒细胞胞外陷阱(NETs),从而保护肝细胞免于纤维化。此外,BA通过阻断nr2f2驱动的HMGB1通路,抑制炎症介质如NLRP3、caspase-1和IL-1β的合成,显著逆转肝纤维化。这些观察结果突出了BA作为肝纤维化可行的治疗候选药物的疗效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
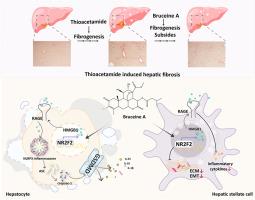
Bruceine A attenuates fibrogenesis and inflammation through NR2F2-regulated HMGB1 inflammatory signaling cascades in hepatic fibrosis
This investigation explored the hepatoprotective capabilities of Bruceine A (BA) and its underlying mechanisms in mitigating hepatic fibrosis. Hepatic stellate cells (HSCs) and mouse primary hepatocytes were treated with TGF-β and subsequently exposed to BA. To assess the effects of BA on the NR2F2-HMGB1 signaling cascade, these cells underwent transfection with a siRNA vector targeting NR2F2. The interaction between NR2F2 and the HMGB1 promoter was elucidated using a dual luciferase assay. In vivo, C57BL/6 mice were treated with thioacetamide (TAA) to induce liver damage, followed by administration of BA. The study found that BA moderated extracellular matrix (ECM) buildup, epithelial-mesenchymal transition (EMT), and inflammatory mediator levels, while concurrently reducing NR2F2 and HMGB1 expression in activated HSCs. Furthermore, BA lessened pyroptosis in hepatocytes, curtailing the inflammatory response. The absence of NR2F2 in HSCs or hepatocytes hindered BA's inhibitory effect on this pathway. It was demonstrated that NR2F2 binds directly to the HMGB1 promoter. Treatment with BA resulted in diminished serum levels of ALT and AST, mitigated damage in hepatic tissues, and decreased the ECM and neutrophil extracellular traps (NETs), thus protecting hepatocytes from fibrosis. Furthermore, BA suppressed the synthesis of inflammatory mediators such as NLRP3, caspase-1, and IL-1β by blocking the NR2F2-driven HMGB1 pathway, markedly reversing hepatic fibrosis. These observations highlight the efficacy of BA as a viable therapeutic candidate for hepatic fibrosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


