螺旋吡喃在多肽合成和光致变色性质的调节中的作用
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
材料的光控触发需要将分子光开关有效地嵌入到更大的分子中。我们在此提出了合成两种用于合成光可切换肽的新构建块,通过一种新颖但尚未报道的方法将螺吡烷作为中心单元嵌入肽骨架中。本文提出的合成方法使我们能够将螺吡烷直接嵌入固相肽合成(SPPS)中,进一步描述所制备的光开关肽的光物理性质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
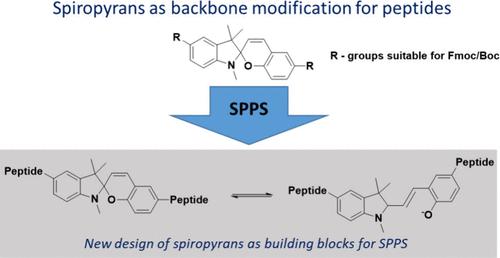
Spiropyran as Building Block in Peptide Synthesis and Modulation of Photochromic Properties
Light-controlled triggering of materials requires efficient embedding of molecular photoswitches into larger molecules. We herein present the synthesis of two new building blocks for the synthesis of photoswitchable peptides, embedding spiropyranes as a central unit into peptide-backbones via a novel, yet unreported approach. The synthesis presented here allows us to embed spiropyranes directly into solid-phase peptide synthesis (SPPS), further describing the resulting photophysical properties of the as-prepared photoswitchable peptides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


