抑制与降解相结合的双靶双机制设计策略
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
胶质母细胞瘤是一种高度侵袭性的脑肿瘤,缺乏有效治疗,5年生存率低。开发新疗法的紧迫性显而易见。哺乳动物雷帕霉素靶蛋白(mTOR)和G1到S相变1基因(GSPT1)在胶质母细胞瘤中过表达,调节重要细胞功能。目前mTOR抑制剂在临床疗效和耐药方面面临挑战。同样,gspt1靶向治疗胶质母细胞瘤在临床试验中也没有进展。研究表明,mTOR抑制与GSPT1降解相结合可以克服耐药性,提高疗效。我们提出了对不同蛋白质共同实施抑制和降解的概念,将抑制剂和降解剂的性质整合到同一个分子中。引入新型双功能分子YB-3-17,有效抑制mTOR并选择性降解GSPT1。作为概念验证研究的工具化合物,YB-3-17提高了选择性,避免了脱靶效应,并选择性地诱导GSPT1降解和mTOR抑制,与单独治疗相比,在肿瘤细胞系中显示出优越的疗效。RNA-seq分析强调了YB-3-17优于mTOR抑制剂治疗的优势。YB-3-17可以安全有效地抑制小鼠肿瘤生长,为胶质母细胞瘤的精准治疗提供了一个有希望的方向,首次尝试将mTOR抑制与GSPT1降解结合起来。这项工作还表明,从概念上讲,将小分子抑制剂和降解剂的特性成功地结合到一个分子中是可能的,从而一举两得。本文章由计算机程序翻译,如有差异,请以英文原文为准。
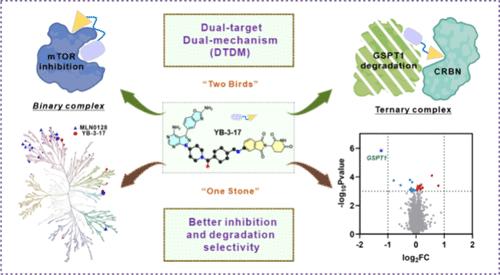
A Dual-Target and Dual-Mechanism Design Strategy by Combining Inhibition and Degradation Together
Glioblastoma, a highly aggressive brain tumor, lacks effective treatment with low 5 year survival rates. Urgency for new therapies is evident. Mammalian targets of rapamycin (mTOR) and G1 to S phase transition 1 gene (GSPT1) are overexpressed in glioblastoma, regulating vital cellular functions. Current mTOR inhibitors face challenges in clinical efficacy and drug resistance. Similarly, GSPT1-targeting therapies have not progressed of glioblastoma in clinical trials. Research studies suggested that combining mTOR inhibition with GSPT1 degradation may overcome resistance and enhance efficacy. We propose the concept of jointly implementing inhibition and degradation on different proteins, integrating the properties of inhibitors and degraders into the same molecule. Introducing YB-3–17, a novel bifunctional molecule, robustly inhibits mTOR and selectively degrades GSPT1. As a tool compound for proof-of-concept studies, YB-3–17 sharpens selectivity, avoiding off-target effects, and selectively induces GSPT1 degradation and mTOR inhibition, showing superior efficacy in tumor cell lines compared to that of standalone therapies. RNA-seq analysis highlights the advantages of YB-3-17 over mTOR inhibitor treatment. YB-3–17 can safely and effectively inhibit tumor growth in mice, offering a promising direction for precision treatment of glioblastoma, representing the first attempt to combine mTOR inhibition with GSPT1 degradation. This work also demonstrates that it is conceptually possible to successfully combine the properties of small molecule inhibitors and degraders into a single molecule, killing two birds with one stone.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


