一种新的双官能团过氧化物串联反应可以方便地合成官能团化的二氢呋喃
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
双功能过氧化物是多用途试剂。在此,我们报道了一种新的过氧烯醛环化模式,该模式在室温DBU碱性条件下与β-酮酯进行独特的解共轭环氧化-醛醇加成-环化级联。该反应可快速构建含相邻季叔碳中心和多个非对映选择性高的官能团的复合二氢呋喃。本文章由计算机程序翻译,如有差异,请以英文原文为准。
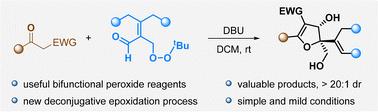
A new tandem reaction of bifunctional peroxides enables the expedient synthesis of functionalized dihydrofurans†
Bifunctional peroxides are versatile reagents. Herein, we report a new annulation mode of peroxy enals that undergo a distinctive cascade reaction with β-keto esters under DBU basic conditions at room temperature, with the cascade specifically involving deconjugative epoxidation and aldol addition/cyclization. The reaction could rapidly construct complex dihydrofurans bearing adjacent quaternary–tertiary carbon centers and multiple functional groups with high diastereoselectivities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


