Ru(II)催化氧化还原-中性碳氢烯烃与乙烯基砜串联环化:利用亚磺酸阴离子作为离去基合成3-亚甲基异吲哚-1- 1
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了Ru(II)催化的n -甲氧基苯酰胺与乙烯基砜的氧化还原-中性碳氢烯烃反应。该反应操作简单,在室温下进行,不需要银添加剂或外部氧化剂,使其适合后期功能化。值得注意的是,我们利用亚硫酸盐阴离子的离去基能力,在室温下通过C-H烯烃/环化/消除序列合成了3-亚甲基异吲哚-1- 1。此外,我们成功地证明了3-亚甲基异吲哚-1- 1的合成通用性,用于构建异吲哚苯并氮杂平核心。本文章由计算机程序翻译,如有差异,请以英文原文为准。
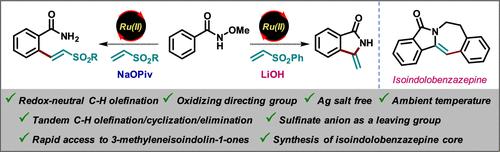
Ru(II)-Catalyzed Redox-Neutral C–H Olefination and Tandem Cyclization with Vinyl Sulfones: Leveraging Sulfinate Anion as a Leaving Group for the Synthesis of 3-Methyleneisoindolin-1-ones
We developed a Ru(II)-catalyzed redox-neutral C–H olefination of N-methoxybenzamides with vinyl sulfones. The reaction is operationally simple and conducted at ambient temperature and does not require silver additives or external oxidants, making it suitable for late-stage functionalization. Notably, we leveraged the leaving group ability of sulfinate anion to synthesize 3-methyleneisoindolin-1-ones through a tandem C–H olefination/cyclization/elimination sequence at ambient temperature. Moreover, we successfully demonstrated the synthetic versatility of 3-methyleneisoindolin-1-one for the construction of an isoindolobenzazepine core.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


