光催化染料降解的还原氧化石墨烯- CeO2纳米复合材料
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
在过去的几十年里,染料的广泛使用造成了相当大的环境污染,对人类健康和生态系统构成了严重威胁。在此背景下,本文重点研究了一种由还原氧化石墨烯(rGO)和氧化铈(CeO2)组成的定制纳米复合材料,以消除亚甲基蓝(MB)染料。制造过程包括一个简单的合成,其中CeO2纳米颗粒通过在还原剂存在的情况下将铈前体盐与氧化石墨烯(GO)混合共沉淀法固定在氧化石墨烯片上。采用x射线衍射(XRD)对复合材料进行了表征,结果表明CeO2和rGO-CeO2的晶粒尺寸分别为21.61 nm和10.74 nm。进一步的FE-SEM分析生动地说明了相对球形的CeO2,它分散在还原氧化石墨烯表面,能量色散x射线光谱(EDX)结果清楚地证明了复合材料中存在碳、铈和氧。此外,傅里叶变换红外光谱(FTIR)证实了rGO-CeO2纳米复合材料的组成,证明了各种含氧基团的存在,包括O- h, C=O和Ce-O键。热重分析(TGA)表明,rGO-CeO2纳米复合材料具有较高的热稳定性,高达400°C时,其重量损失仅为21.88%。此外,rGO-CeO2纳米复合材料在紫外光照射(λ = 254 nm)的最佳条件下,在pH为9、浓度为20 ppm的MB染料溶液中,催化剂用量为7 mg,在80 min内对MB染料的降解率达到94.92%。相比之下,原始rGO和CeO2在100和90分钟内分别仅达到32.27%和38.48%。对光催化过程的动力学进行了细致的研究,发现它们遵循伪二级动力学模型,表明MB染料在降解之前被化学吸附到纳米复合材料上。在最佳条件下评估了纳米复合材料的稳定性,即使在第4次循环中也显示出强劲的MB降解(78%)。总的来说,本研究的成功结果表明,使用rGO-CeO2纳米复合材料降解废水中的MB染料具有很大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
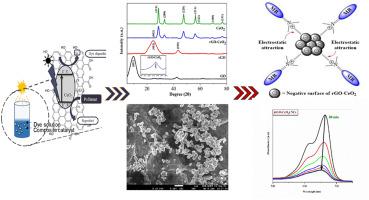
Reduced graphene oxide – CeO2 nanocomposites for photocatalytic dye degradation
Over the past few decades, the widespread use of dyes has led to considerable environmental pollution, posing serious threats to human health and ecosystems. In this context, here focus on a tailored nanocomposite comprising reduced graphene oxide (rGO) and cerium oxide (CeO2) to eradicate methylene blue (MB) dye. The fabrication process involves a facile synthesis wherein CeO2 nanoparticles are anchored onto rGO sheets through co-precipitation treatment by mixing a precursor salt of cerium with graphene oxide (GO) in presence of reducing agent. The resulting nanocomposites were characterized using X-ray diffraction (XRD) analysis which confirms the crystallite size of CeO2 and rGO-CeO2 determined to be 21.61 nm and 10.74 nm respectively. Further FE-SEM analysis vividly illustrates the relatively spherical form of CeO2, which is dispersed on the rGO surface and energy dispersive X-ray spectroscopy (EDX) results provide clear evidence of the presence of carbon, cerium and oxygen in the composite. Additionally, Fourier-transform infrared spectroscopy (FTIR) confirms the composition of the rGO-CeO2 nanocomposites demonstrating the existence of various oxygen-containing groups, including O–H, C=O and Ce–O bonds. Thermogravimetric analysis (TGA) highlights that the rGO-CeO2 nanocomposites exhibits higher thermal stability with only 21.88 % weight loss up to 400 °C. Moreover, the photocatalytic activity of rGO-CeO2 nanocomposite exhibits a remarkable 94.92 % degradation of MB dye within 80 min under optimal conditions of UV light irradiation (λ = 254 nm), with a catalyst dosage of 7 mg in a 20 ppm of MB dye solution at pH 9. In contrast, pristine rGO and CeO2 achieved only 32.27 % and 38.48 % in 100 and 90 min respectively. The kinetics of photocatalysis process were meticulously studied and revealed that they followed pseudo-second order kinetic model suggesting chemisorption of MB dye onto nanocomposites prior to degradation. The stability of the nanocomposite was assessed under optimal conditions, demonstrating robust MB degradation (78 %) even up to the 4th cycle. Overall, the successful outcomes of this study show significant potential for degrading MB dye in wastewater by using the rGO-CeO2 nanocomposite.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


