新型环保型喹啉酮衍生物水溶性低碳钢缓蚀剂:合成、缓蚀效果及DFT分析
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
介绍了以3-(2-苯腙)喹啉-2,4(1H,3H)二酮1为原料,快速合成4-亚胺-3-(2-苯腙)-3,4-二氢喹啉-2(1H)- 1 (IQ)的新方法。采用失重法(Wt-L)和电化学方法(如动电位极化法(Pd-P)和电化学阻抗谱法(EIS)评价了IQ对低碳钢(LCS)在酸性环境中的缓蚀效果。结果表明,IQ的最佳浓度为30×10−3 Mm,在此浓度下,其抑菌率最高,约为93.2%。IQ作为一种混合型抑制剂的性质证明了它同时抑制阳极和阴极反应的能力,主要是作为阳极抑制剂。IQ在LCS表面的吸附遵循Langmuir吸附模型,表明其为单层吸附机制。此外,EIS研究结果表明,IQ在LCS表面金属/电解质界面处形成保护层,防止其在酸性介质中溶解。在LCS浸泡两天后,对含有IQ的腐蚀溶液的进一步分析表明,通过紫外/可见光谱法证实了Fe-IQ复合物的形成。利用原子力显微镜(AFM),我们证实了在LCS表面形成一层IQ保护层。此外,IQ的硅研究揭示了在酸性条件下与LCS表面相互作用的活性位点。这些发现强调了IQ作为酸性环境中LCS的有效缓蚀剂的潜力,通过在金属表面形成稳定的保护膜来提供重要的保护。本文章由计算机程序翻译,如有差异,请以英文原文为准。
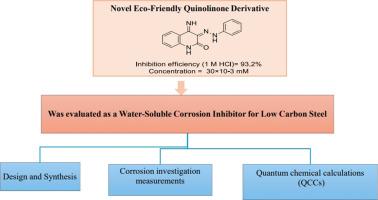
Novel eco-friendly quinolinone derivative as a water-soluble corrosion inhibitor for low carbon steel: Synthesis, inhibitive efficacy, and DFT analysis
A novel and rapid synthetic route for producing 4-imino-3-(2-phenylhydrazono)-3,4-dihydroquinolin-2(1H)-one (IQ) from 3-(2-phenylhydrazono)quinoline-2,4(1H,3H)‑dione 1 is introduced. The inhibitory effectiveness of IQ in mitigating the corrosion of low carbon steel (LCS) in acidic environments was evaluated using weight loss (Wt-L) and electrochemical methods, such as potentiodynamic polarization (Pd-P) and electrochemical impedance spectroscopy (EIS). The optimum concentration of IQ was determined to be 30×10−3 Mm, at which it exhibited the highest inhibition efficiency of approximately 93.2 %. The nature of IQ as a mixed-type inhibitor was demonstrated by its ability to inhibit both anodic and cathodic reactions, primarily acting as an anodic inhibitor.
The adsorption of IQ onto the LCS surface followed the Langmuir adsorption model, indicating a monolayer adsorption mechanism. Moreover, EIS findings suggest that IQ forms a protective layer on the LCS surface at the metal/electrolyte interface, preventing its dissolution in the acidic medium. Further analysis of the corrosive solution containing IQ after two days of LCS immersion showed the formation of Fe-IQ complex, confirmed by ultraviolet/visible spectroscopy. Using atomic force microscopy (AFM), we confirmed that a protective layer of IQ forms on the surface of LCS. Additionally, in silico studies of IQ revealed the active sites responsible for its interaction with the LCS surface under acidic conditions. These findings underscore the potential of IQ as an effective corrosion inhibitor for LCS in acidic environments, providing significant protection by forming a stable, protective film on the metal surface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


