不对称配体立体异构体阴离子模板自组装形成的复杂低对称Ag6L4胶囊
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
金属有机笼和胶囊基于其离散的空心结构呈现出特定的空间功能。为了获得类似酶的不对称或复杂的结构,它们被用两种或两种以上不同的配体去对称修饰。有必要建立新的策略,以一种简单的方式,只使用一种配体,这是不同于混合配体方法的结构去对称。本研究开发了一种利用不对称大环配体苯并咪唑[3]芳烃形成可互换立体异构体的策略。单晶x射线衍射分析表明,与四氟硼酸银组装的异构体形成了结构复杂的构象异位的Ag6L4胶囊。胶囊中的6个Ag离子不对称,导致配位几何形状明显不同。值得注意的是,在固体和溶液状态下,胶囊通过多点C-H···F-B氢键封装了一个四氟硼酸盐阴离子,这表明适当大小和形状的阴离子可以作为胶囊形成的模板。这些结果表明,使用异构和不对称配体是由单个配体构建高度不对称超分子结构的有效方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
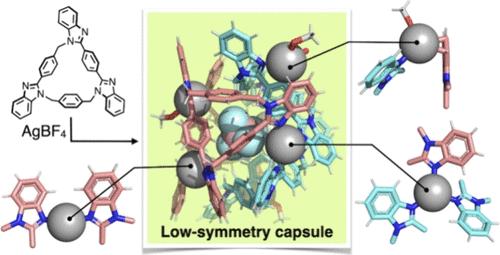
Intricate Low-Symmetry Ag6L4 Capsules Formed by Anion-Templated Self-Assembly of the Stereoisomers of an Unsymmetric Ligand
Metal–organic cages and capsules exhibit space-specific functions based on their discrete hollow structures. To acquire enzyme-like asymmetric or intricate structures, they have been modified by desymmetrization with two or more different ligands. There is a need to establish new strategies that can desymmetrize structures in a simple way using only one type of ligand, which is different from the mixed-ligand approach. In this study, a strategy was developed to form interconvertible stereoisomers using the unsymmetric macrocyclic ligand benzimidazole[3]arene. Single-crystal X-ray diffraction analysis revealed that the isomers assembled with silver tetrafluoroborate afforded a conformationally heteroleptic Ag6L4 capsule with an intricate structure. The six Ag ions in the capsule were desymmetrized, resulting in significantly different coordination geometries. Remarkably, the capsule encapsulates a single tetrafluoroborate anion via multipoint C–H···F–B hydrogen bonds in both the solid and solution states, suggesting that anions of appropriate size and shape can act as a template for the capsule formation. These results demonstrate that the use of isomerizable and unsymmetric ligands is the effectiveness of constructing highly dissymmetric supramolecular structures from a single ligand.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


