Withaferin-A诱导的Vimentin S56磷酸化解离NEDD9信号环,使进展性转移性黑色素瘤退化为肺腺癌。
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
转移是一种复杂而隐匿的疾病类型,涉及多种信号通路,这对理解疾病的发病机制具有重要意义。转移性癌症的治疗失败率通常很高,这是由于癌细胞对邻近器官的侵袭性适应。神经前体细胞表达的发育下调蛋白9 (NEDD9)通过调控多种转移事件,在转移事件中发挥关键作用。这些蛋白之间的相互作用是促进转移进展所必需的。Withaferin A (WFA)是一种天然药物,已知可靶向Vimentin诱导抗肿瘤潜能。但其确切的分子机制尚未阐明。我们假设,Vimentin-NEDD9信号通路对于转移进展是必要的,并且用有效的pharamacophore WFA靶向这种交织的信号回路可以阻止黑色素瘤向肺的转移进展。为了阐明这一点,我们通过定量逆转录聚合酶链反应(qRT-PCR)、免疫印迹和免疫组织化学进行了基因表达测定。通过共免疫沉淀、免疫荧光、共定位和接近结扎试验评估相互作用信号。磷酸化研究是通过位点定向诱变产生的磷酸化特异性突变体构建体的转染。通过迁移、侵袭和免疫印迹分析,WFA诱导细胞行为改变。B16F10诱导小鼠转移性黑色素瘤模型,检测WFA诱导的NEDD9-Vimentin表达及抗转移能力。研究结果表明,NEDD9-Vimentin蛋白水平升高及蛋白间相互作用与体外和体内黑色素瘤向肺转移进展呈正相关,可作为治疗靶点。从药理学上讲,WFA不仅通过诱导Vimentin特异性丝氨酸56磷酸化,还通过解离NEDD9信号环来阻止上皮-间质转化(EMT)和随后的转移事件,从而延长该复合物的活性。最终,相关转移基因E-Cadherin, N-Cadherin, SNAIL, MMP-2和MMP-9的调节导致转移性黑色素瘤进展到肺部的消退。该研究证实了WFA诱导的S56磷酸化对于突变NEDD9-Vimentin转移信号复合物以逆转侵袭性转移性黑色素瘤是必要的。调查强调更机械的WFA方法。了解和靶向肿瘤微环境中的这种综合机械输入将是对抗转移的更好的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
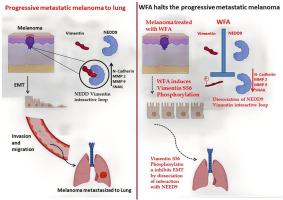
Withaferin-A induced vimentin S56 phosphorylation dissociates NEDD9 signaling loop to regress progressive metastatic melanoma into lung adenocarcinoma
Metastasis is complex and insidious type of disease involves multiple signaling nexus, which have implications in understanding disease pathogenesis. Treatment failure for metastatic cancer is frequently high due to aggressive adaptation of cancerous cells to invade to neighboring organs. Cytoskeleton intermediate filamentous protein Vimentin and scaffolding protein Neural precursor cell expressed Developmentally Down-regulated protein 9 (NEDD9) play a key role in metastatic events by regulating multiple metastatic events. Interaction between these proteins is necessary to promote metastatic progression. Withaferin A (WFA), a natural pharamacophore, known to target Vimentin to induce antitumor potential. However exact molecular mechanism still yet to be elucidated. We hypothesize, Vimentin-NEDD9 signaling nexus is necessary for metastatic progression and targeting this interwoven signaling loop with effective pharamacophore WFA halts metastatic progression of melanoma into lung. To elucidate the same, we carried out gene expression measurement through quantitative Reverses Transcription Polymerase Chain Reaction (qRT-PCR), Immunoblot and Immunohistochemistry. Assessment of interactive signaling by Co-immunoprecipitation, Immunofluorescence, Co-localization and Proximity ligation assay. Phosphorylation studies through transfection of phospho specific mutant constructs generated through site directed mutagenesis. WFA induced cellular behavioral changes by migration, invasion assays and Immunoblot analysis. The B16F10 induced mouse metastatic melanoma model to asses NEDD9-Vimentin expression and anti-metastasis induced by WFA. The results postulates, elevated levels and interaction between NEDD9-Vimentin proteins, have positive correlation in metastatic progression of melanoma into lung in both in-vitro and in-vivo condition, establishing it as therapeutic target. Pharmacologically, WFA targets this complex by extending its activity by not only inducing specific Serine 56 phosphorylation of Vimentin, also dissociates NEDD9 signaling loop to halt Epithelial-mesenchymal transition (EMT) and subsequent metastatic events. Eventually, modulation of the relevant metastatic genes E-Cadherin, N-Cadherin, SNAIL, MMP-2 & MMP-9 resulted in regression of metastatic melanoma progression to lung. The study validates WFA induced S56 phosphorylation is necessary to abrupt the NEDD9-Vimentin metastatic signaling complex to regress aggressive metastatic melanoma. The investigation emphasized more mechanistic approach of WFA. Understanding and targeting such integrative mechanical input in the tumor microenvironment will be a better therapeutic strategy to combat metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


