打开伤口愈合的潜力:一个集成的硅蛋白质组学和体内分析Tacorin,一个生物活性蛋白组分,从Ananas comosus (L.)稳定。茎
IF 2.3
4区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Proteins and proteomics
Pub Date : 2024-11-26
DOI:10.1016/j.bbapap.2024.141060
引用次数: 0
摘要
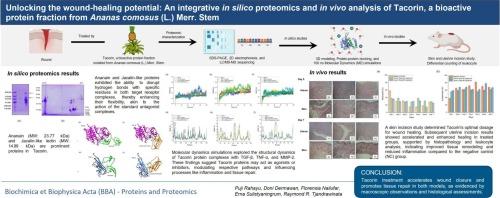
塔可林是一种从菠萝茎(Ananas comosus)中提取的生物活性蛋白,已成为一种有前景的伤口愈合治疗剂。本研究采用综合方法,结合硅蛋白质组学和体内研究,揭示他可林伤口愈合特性的分子机制。在硅蛋白组学领域,通过LC/MS-MS蛋白测序对Tacorin的组成进行了分析,发现其主要成分为ananain (23.77 kDa)和jacalin样凝集素(14.99 kDa)。分子蛋白对接模拟揭示了Tacorin成分与伤口愈合关键调节因子(包括TGF-β、TNF-α和MMP-2)之间有利的相互作用。计算的自由结合能表明Tacorin蛋白与其靶受体之间具有很强的结合亲和力。具体而言,ananain与TGF-β的结合亲和力为- 12.2 kcal/mol,表明其可能是TGF-β介导的信号传导的有效激活剂,而jacalin样凝集素与TNF-α的结合亲和力为- 8.7 kcal/mol。随后的100ns分子动力学(MD)模拟提供了对他可林受体复合物的动态行为和稳定性的深入了解,揭示了他可林治疗效果的分子决定因素。作为计算机分析的补充,体内研究通过皮肤和子宫切口模型评估了他可林在伤口愈合中的功效。宏观观察和组织学评估证明,他可林治疗在两种模型中都能加速伤口愈合并促进组织修复。总的来说,这项研究提供了令人信服的证据,证明了他可林在伤口愈合中的治疗潜力,并强调了阐明其分子机制对进一步开发和临床转化的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unlocking the wound-healing potential: An integrative in silico proteomics and in vivo analysis of Tacorin, a bioactive protein fraction from Ananas comosus (L.) Merr. Stem
Tacorin, a bioactive protein fraction derived from pineapple stem (Ananas comosus), has emerged as a promising therapeutic agent for wound healing. This study employs an integrated approach, combining in silico proteomics and in vivo investigations, to unravel the molecular mechanisms underlying Tacorin's wound healing properties. In the domain of in silico proteomics, the composition of Tacorin is elucidated through LC/MS-MS protein sequencing, revealing ananain (23.77 kDa) and Jacalin-like lectin (14.99 kDa) as its predominant constituents. Molecular protein-protein docking simulations unveil favorable interactions between Tacorin's components and key regulators of wound healing, including TGF-β, TNF-α, and MMP-2. The calculated free binding energies indicate strong binding affinities between Tacorin proteins and their target receptors. Specifically, ananain demonstrates a binding affinity of −12.2 kcal/mol with TGF-β, suggesting its potential as a potent activator of TGF-β-mediated signaling, while Jacalin-like lectin exhibits the most favorable binding affinity of −8.7 kcal/mol with TNF-α. Subsequent 100 ns molecular dynamics (MD) simulations provide insights into the dynamic behavior and stability of Tacorin-receptor complexes, shedding light on the molecular determinants of Tacorin's therapeutic effects. Complementing the in silico analyses, in vivo studies evaluate Tacorin's efficacy in wound healing using skin and uterine incision models. Tacorin treatment accelerates wound closure and promotes tissue repair in both models, as evidenced by macroscopic observations and histological assessments. Overall, this study provides compelling evidence of Tacorin's therapeutic potential in wound healing and underscores the importance of elucidating its molecular mechanisms for further development and clinical translation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
0.00%
发文量
55
审稿时长
33 days
期刊介绍:
BBA Proteins and Proteomics covers protein structure conformation and dynamics; protein folding; protein-ligand interactions; enzyme mechanisms, models and kinetics; protein physical properties and spectroscopy; and proteomics and bioinformatics analyses of protein structure, protein function, or protein regulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


