探究电解质离子对镍基金属有机框架电化学性能的影响
IF 4.7
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
了解电解质离子尺寸对电化学性能的影响对于推动基于 MOF 的卓越装置的开发至关重要。纳米片型镍金属有机框架(Ni-BDC)已成为下一代超级电容器的特殊材料。为此,我们通过基本溶热法研究了 1,4-苯二甲酸镍(Ni-BDC)的储能性能。本研究的主要目的是阐明水性电解质中不同阳离子成分对 Ni-BDC 电化学效率的影响。Ni-BDC 的计算比电容值在 1 A/g 时依次增加了 204 F/g(LiOH)< 290 F/g(NaOH)< 1526 F/g(KOH),这是因为水合球半径最小,K+ 离子的离子迁移率更大。该材料在 KOH 电解液中的电化学性能更好,电容保持率达 87%。此外,还利用活性炭(AC)作为负极,纳米片状 Ni-BDC 作为正极,构建了一个不对称超级电容器(ASSC)装置。该装置在 1.4 V 电位窗口内工作,在电流密度为 1 A/g 时的电容为 39.4 F/g。在 8 A/g 的电流密度下,ASSC 器件在 4000 次充放电循环中保持了 85% 的循环稳定性。这项研究强调了选择最佳电解质对显著提高 Ni-BDC 电极材料电化学性能的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
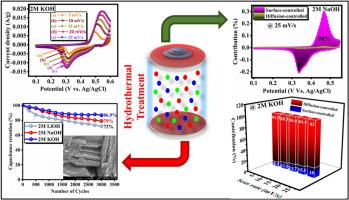
Probing the effect of electrolyte ions on the electrochemical performance of nickel-based metal-organic frameworks
Understanding the influence of electrolyte ions dimension on electrochemical performance is essential for advancing the development of superior MOF-based devices. Nanosheet-type nickel metal-organic frameworks (Ni-BDC) have emerged as exceptional materials for next-generation supercapacitors. To address this, we investigate the energy storage performance of nickel-1,4-benzene dicarboxylate (Ni-BDC) via an elementary solvothermal approach. The primary objective of the present investigation was to elucidate the influence of distinct cationic components within aqueous electrolytes on the electrochemical efficiency of Ni-BDC. The electrochemical examination was performed using a three-electrode setup in different aqueous electrolytes, comprising LiOH, NaOH, and KOH, each with a concentration of 2 M. The calculated specific capacitance value of Ni-BDC increases in the order of 204 F/g (for LiOH) < 290 F/g (for NaOH) < 1526 F/g (for KOH) at 1 A/g due to the lowest hydration sphere radius, greater ionic mobility of K+ ion. The material performed better electrochemically in KOH electrolyte with 87 % capacitance preservation. Additionally, an asymmetric supercapacitor (ASSC) device was built using activated carbon (AC) as the negative electrode and nanosheet-like Ni-BDC as the positive electrode. The device, operating within a 1.4 V potential window, demonstrated a capacitance of 39.4 F/g at a current density of 1 A/g. The ASSC device maintained 85 % of its cyclic stability over 4000 charge-discharge cycles at a current density of 8 A/g. This study underscores the vital importance of selecting the optimal electrolyte to significantly enhance the electrochemical performance of Ni-BDC electrode material.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


