沙福甙通过异构靶向 CaMKII-δ 调节自噬-溶酶体通路,改善高房颤动性脑梗塞(HFpEF)病情
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
射血分数保留型心力衰竭(HFpEF)表现复杂,给全球医疗保健系统带来了巨大挑战。HFpEF 表现为左心室射血分数正常或接近正常、心脏舒张功能障碍以及以炎症和氧化应激受损为特征的代谢特征。迄今为止,针对高频心衰的有价值的药物靶点报道很少。在这里,我们发现甘草中的一种活性成分--五味子苷对持续输注血管紧张素 II(AngII)诱导的心脏重塑有显著的保护作用,而血管紧张素 II 会导致高频心衰表型。从机理上讲,七叶皂苷能在体外和体内模型中改善溶酶体功能障碍,从而激活自噬。基于蛋白质组和磷酸蛋白组的生物信息学分析表明,Ca2+/钙调蛋白依赖性蛋白激酶II(CaMKII)是沙夫苷的潜在靶点。研究证实,七叶皂苷能通过靶向ATP结合位点附近一个独特的活性口袋,异生介导CaMKII-δ构象,从而抑制蛋白磷酸化,调控溶酶体自噬途径。因此,沙夫苷是第一个被发现通过异位抑制作用抑制 CaMKII-δ 活性的小分子,为缓解高频血友病患者的心脏代谢失衡提供了一种新的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
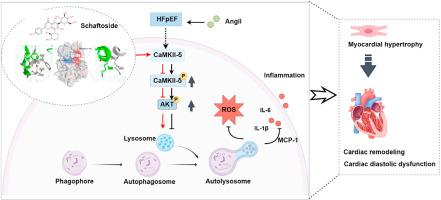
Schaftoside improves HFpEF through regulation the autophagy-lysosome pathway by allosterically targeting CaMKII-δ
Heart failure with preserved ejection fraction (HFpEF) presents a significant challenge to global healthcare systems due to its complex presentation. HFpEF presents with a normal or near-normal left ventricular ejection fraction, cardiac diastolic dysfunction, and a metabolic profile characterized by impaired inflammation and oxidative stress. There have been few valuable drug targets reported for HFpEF to date. Here, we discovered that schaftoside, an active component from licorice, has a significant protective effect on the cardiac remodeling induced by continuous infusion of angiotensin II (AngII), which leads to the HFpEF phenotype. Mechanistically, schaftoside has demonstrated the ability to ameliorate lysosomal dysfunction in both in vitro and in vivo models, thereby activating autophagy. Bioinformatic analyses based on proteome and phosphoproteome suggested that Ca2+/calmodulin-dependent protein kinase II (CaMKII) was a potential target for schaftoside. It was confirmed that schaftoside allosterically mediated CaMKII-δ conformation via targeting a unique active pocket near the ATP-binding site to inhibit protein phosphorylation and regulate the lysosomal autophagy pathway. Therefore, schaftoside represents the first small molecule identified to inhibit CaMKII-δ activity through allosteric inhibition, providing a novel candidate for alleviating cardiac metabolic imbalance in HFpEF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


