5-氮杂-2′-脱氧胞苷(地西他滨)可增加头颈部鳞状细胞癌的癌睾抗原表达,并改变免疫检查点的表达,尤其是 CD39 阳性 CD8 和 CD4 T 细胞的表达
IF 4.8
2区 医学
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
摘要
头颈部鳞状细胞癌(HNSCC)患者的免疫疗法失败表明,增强适应性免疫杠杆作用的需求尚未得到满足。免疫原癌睾丸抗原(CTA)的表达以及淋巴细胞的分化和功能受DNA甲基化的调控。因此,通过5-氮杂-2′-脱氧胞苷(DAC)抑制DNA-甲基转移酶的表观遗传疗法是一种很有前景的免疫辅助疗法。我们研究了 DAC 对 HNSCC 细胞系中 CTA 表达和增殖能力的影响,以及对口咽鳞状细胞癌(OPSCC)患者和健康供体淋巴细胞中 12 种免疫检查点分子(ICM)表达的影响。在淋巴细胞中,观察到 ICM 重排发生了明显变化,受供体类型和亚群的影响。在 CD39+ CD4 和 CD8 T 细胞上,共刺激 ICM GITR 和 OX40 的表达呈剂量依赖性增加,而在 CD39- CD4 T 细胞上则有所减少。PD1 的表达主要在 CD39+ CD8 T 细胞上增加,而在 CD39- CD4 T 细胞上减少。CD27的表达主要在CD8 T细胞中减少,但在CD39- CD4 T细胞中增加,而ICOS的表达在CD4和CD8 T细胞的CD39+和CD39-亚群中均降低。我们建议将低剂量 DAC 治疗作为免疫疗法的辅助手段。本文章由计算机程序翻译,如有差异,请以英文原文为准。
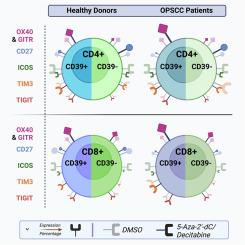
5-Aza-2′-deoxycytidin (Decitabine) increases cancer-testis antigen expression in head and neck squamous cell carcinoma and modifies immune checkpoint expression, especially in CD39-positive CD8 and CD4 T cells
Failure of immunotherapy in head and neck squamous cell carcinoma (HNSCC) patients represents an unmet need to augment leverage of adaptive immunity. Immunogenic cancer-testis antigen (CTA) expression as well as lymphocyte differentiation and function are regulated by DNA methylation. Therefore, epigenetic therapy via inhibition of DNA-Methyltransferases by 5-Aza-2′-deoxycytidine (DAC) serves a promising adjuvant in immunotherapy.
We investigated the effects of DAC on CTA expression and proliferative capacity in HNSCC cell lines and on the expression of 12 immune checkpoint molecules (ICM) on lymphocytes of oropharyngeal squamous cell carcinoma (OPSCC) patients and healthy donors.
In all cell lines CTA were upregulated accompanied by decreased proliferation. In lymphocytes pronounced alterations of the ICM repertoire were observed, influenced by donor type and subpopulation. On CD39+ CD4 and CD8 T cells, the expression of co-stimulatory ICM GITR and OX40 increased dose dependently, whereas expression decreased on CD39- CD4 T cells. PD1 expression increased primarily on CD39+ CD8 T cells and decreased on CD39- CD4 T cells. CD27 expression decreased primarily in CD8 T cells, but increased in CD39- CD4 T cells, whereas ICOS expression was lowered in both CD39+ and CD39- subsets of CD4 as well as CD8 T cells.
DAC treatment increased immunogenicity and decreased proliferation in HNSCC cells while enhancing expression of co-stimulatory ICM GITR and OX40. We propose low dose DAC treatment as a adjuvant to immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neoplasia
医学-肿瘤学
CiteScore
9.20
自引率
2.10%
发文量
82
审稿时长
26 days
期刊介绍:
Neoplasia publishes the results of novel investigations in all areas of oncology research. The title Neoplasia was chosen to convey the journal’s breadth, which encompasses the traditional disciplines of cancer research as well as emerging fields and interdisciplinary investigations. Neoplasia is interested in studies describing new molecular and genetic findings relating to the neoplastic phenotype and in laboratory and clinical studies demonstrating creative applications of advances in the basic sciences to risk assessment, prognostic indications, detection, diagnosis, and treatment. In addition to regular Research Reports, Neoplasia also publishes Reviews and Meeting Reports. Neoplasia is committed to ensuring a thorough, fair, and rapid review and publication schedule to further its mission of serving both the scientific and clinical communities by disseminating important data and ideas in cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


