利用 Cu2O 微珠催化合成具有生物活性的 1,4-二取代 1,2,3-三唑连环硫代氨基甲酮衍生物,增强抗菌和抗氧化活性
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究重点关注 1,4-二取代 1,2,3-三唑连环硫代氨基甲酮衍生物的抗菌和抗氧化特性的设计、合成和评估。合成涉及一个多步骤过程,首先从三种苯甲醛衍生物(4-羟基苯甲醛、香兰素和水杨醛)制备炔衍生物,然后使用花状 Cu2O 微珠作为催化剂。评估了合成化合物对四种细菌菌株的抗菌活性:以环丙沙星(CIP)为参照物,在 10 至 80 mg/mL 的浓度范围内,对大肠杆菌(E. coli)、铜绿假单胞菌(P. aeruginosa)、枯草杆菌(B. subtilis)和金黄色葡萄球菌(S. aureus)进行了抗菌活性评价。在这些衍生物中,化合物 3c,(Z)-2-(4-((1-苄基-1H-1,2,3-三唑-4-基)甲氧基)-3-甲氧基亚苄基)肼-1-硫代甲酰胺对大肠杆菌的抗菌活性最强,在 80 毫克/毫升的浓度下,抑菌区为 22±0.1 毫米。化合物 3a,即 (Z)-2-(4-((1-苄基-1H-1,2,3-三唑-4-基)甲氧基)亚苄基)肼-1-硫代甲酰胺,对枯草杆菌的抗菌效力最高,抑菌区为 21±0.4 毫米。包括 DPPH 和 ABTS 在内的抗氧化试验显示,化合物 3c 的 IC50 值最低(分别为 2 ± 0.4 μg/mL 和 160±0.1 μg/mL),表明其具有很强的抗氧化活性。这些结果表明,Cu2O 催化合成和化学取代 1,4-二取代 1,2,3-三唑连环硫代氨基甲酮衍生物可显著提高其抗菌和抗氧化活性,使其成为有希望的候选治疗药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
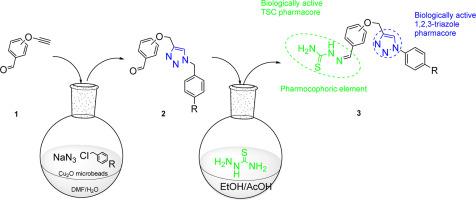
Synthesis of bioactive 1,4-disubstituted 1,2,3-triazole-linked Thiosemicarbazone derivatives using Cu2O microbeads catalysis for enhanced antibacterial and antioxidant activities
This study focuses on the design, synthesis and evaluation of antibacterial and antioxidant properties of 1,4-disubstituted 1,2,3-triazole-linked thiosemicarbazone derivatives.The synthesis involved a multistep process, beginning with the preparation of alkyne derivatives from three benzaldehyde derivatives (4-hydroxybenzaldehyde, vanillin, and salicylaldehyde) and using flower-like Cu2O microbeads as a catalyst. The synthesized compounds were evaluated for antibacterial activity against four bacterial strains: Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Bacillus subtilis (B. subtilis), and Staphylococcus aureus (S. aureus), at concentrations ranging from 10 to 80 mg/mL, using ciprofloxacin (CIP) as a reference. Among the derivatives, Compound 3c, (Z)-2-(4-((1-benzyl-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzylidene)hydrazine-1-carbothioamide,exhibited the most potent antibacterial activity against E. coli, with a zone of inhibition of 22±0.1 mm at 80 mg/mL. Compound 3a, (Z)-2-(4-((1-benzyl-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)hydrazine-1-carbothioamide, showed the highest efficacy against B. subtilis, with an inhibition zone of 21±0.4 mm. Antioxidant assays, including DPPH and ABTS, revealed Compound 3c to have the lowest IC50 values (2 ± 0.4 μg/mL and 160±0.1 μg/mL, respectively), indicating strong antioxidant activity. These results demonstrate that Cu2O-catalyzed synthesis and chemical substitution in 1,4-disubstituted 1,2,3-triazole-linked thiosemicarbazone derivatives significantly enhance their antibacterial and antioxidant activities, making them promising candidates for therapeutic development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


