单金属掺杂的 Al12N12 富勒烯类笼作为载体在各种溶剂中定向输送姜酚
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们利用密度泛函理论(DFT)研究了负载在原始氮化铝(Al12N12)富勒烯类笼上的姜酚(GGL)在水、甲醇和二氯甲烷中的传递,以探索其治疗癌症的潜力。在 CAM-B3LYP 方法中,GGL 通过羟基和羰基在 Al12N12 表面的化学吸附导致水相中的偶极矩和结合能(Ebin)比甲醇和二氯甲烷相中的偶极矩和结合能(Ebin)更高。这一吸附过程导致所有配合物的电子结构发生轻微变化。吸附分析表明,与羰基不同,GGL 的羟基与 Al11BN12 中的 B 原子、Al11MgN12 中的 Mg 原子以及 Al11GaN12 富勒烯笼中的 Ga 原子有很强的相互作用。与 Al11GaN12 相比,Al11MgN12 和 Al11BN12 类富勒烯笼的结合能较弱,因此解吸时间较短。这有利于 GGL 的输送并增加其偶极矩,从而提高其溶解度。此外,Al11MgN12 上负载的 GGL 增加了硬度、电负性和亲电性,同时降低了软度,这表明其稳定性和相互作用能力得到了增强。此外,与 Al11GaN12 和 Al11BN12 笼相比,Al11MgN12 的能隙发生了显著变化,因此作为生物传感器,Al11MgN12 对 GGL 分子具有很高的灵敏度。理论红外(IR)光谱计算表明,由于 GGL 吸附在类富勒烯笼上,其振动频率发生了变化。总之,研究结果表明,GGL负载的Al11MgN12在改善生物应用药物输送系统的溶解性和药效方面具有广阔的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
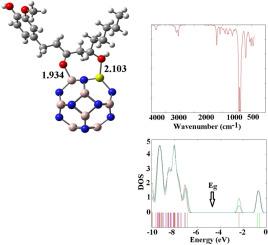
Single-metal doped Al12N12 fullerene-like cages as carriers for targeted gingerol delivery in various solvents
We investigated the delivery of gingerol (GGL) loaded onto pristine and B-, Mg-, and Ga-doped aluminum nitride (Al12N12) fullerene-like cages in water, methanol, and dichloromethane phases using density functional theory (DFT) to explore its potential for cancer treatment. The chemisorption of GGL via hydroxyl and carbonyl groups on the Al12N12 surface resulted in an enhanced the dipole moment and binding energy (Ebin) in the water phase compared to methanol and dichloromethane phases at the CAM-B3LYP method. This adsorption process resulted in slight shifts in the electronic structure of all the complexes. Adsorption analysis revealed that the hydroxyl group of GGL, unlike its carbonyl group, exhibits strong interactions with the B atom in Al11BN12, the Mg atom in Al11MgN12, and the Ga atom in Al11GaN12 fullerene-like cages. The Al11MgN12 and Al11BN12 fullerene-like cages exhibit weaker binding energies compared to Al11GaN12, resulting in shorter desorption times. This facilitates the delivery of GGL and increases its dipole moment, thereby enhancing its solubility. Additionally, GGL loaded onto Al11MgN12 increases hardness, electronegativity, and electrophilicity while decreasing softness, indicating enhanced stability and interaction capabilities. Moreover, Al11MgN12 exhibits high sensitivity to GGL molecules as a biosensor, owing to significant shifts in its energy gap compared to Al11GaN12 and Al11BN12 cages. Theoretical infrared (IR) spectroscopy calculations indicate changes in vibration frequencies due to GGL adsorption onto fullerene-like cages. Overall, the findings suggest that GGL-loaded Al11MgN12 has promising potential for improving solubility and efficacy in drug delivery systems for biological applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


