含杂环的环三唑膦的设计、合成和光谱表征
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究描述了一种含有螺环 2,2′-二氧联苯基团和 2-巯基-1-甲基咪唑(甲巯咪唑)或 2-巯基噻唑啉(MTZ)分子的环三唑膦的合成。这些螺(在双官能团配体中,两端与同一磷结合)环三膦唑衍生物(6、7a)分两步合成。第一步,单取代磷杂蒽化合物 2,4,4,6,6-五氯-2-(2-巯基-1-甲基咪唑基)环 2λ5、4λ5、6λ5、三磷杂蒽;N4P3Cl5C3S2H5 (4),以及 2,4,4,6,6-五氯-2-(2-巯基-噻唑啉基)环 2λ5, 4λ5, 6λ5, 三磷杂三烯;N4P3Cl5C4S2H4(5)]是由六氯环三磷氮杂三烯(三聚体,N3P3Cl6,1)与甲巯咪唑(2)或 MTZ(3)在四氢呋喃(THF)中,在金属钠存在下,于氩气气氛中反应制备而成。第二步,在碳酸钾(K2CO3)存在下,在丙酮中将 4 和 5 与 2,2′-二羟基联苯(8)反应,得到单螺 2-巯基噻唑啉基(7a)和二螺 2-巯基-1-甲基咪唑烷基übstituted(6)磷苯衍生物。利用元素分析、傅立叶变换红外光谱、1H、13C 和 31P NMR 光谱对所有获得的化合物进行了结构表征。本研究首次报道了化合物 5-7a。本文章由计算机程序翻译,如有差异,请以英文原文为准。
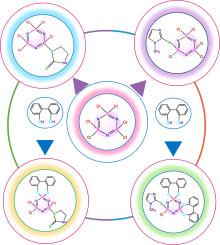
Design, synthesis and spectral characterization of cyclotriphosphazenes including heterocyclic rings
In this study, described the synthesis on a cyclotriphosphazene containing the spirocyclic 2,2′-dioxybiphenyl group and 2-mercapto-1-methylimidazole (methimazole) or 2-mercaptothiazoline (MTZ) moieties. These spiro(in difunctional ligands, both ends are bound to the same phosphorus) cyclotriphosphazene derivatives (6, 7a) were synthesized in two steps. In first step; mono substituted phosphazene compounds 2,4,4,6,6-pentachloro-2-(2-mercapto-1-methylimidazolyl) cyclo2λ5, 4λ5, 6λ5, triphosphazatriene; N4P3Cl5C3S2H5 (4), and 2,4,4,6,6-pentachloro-2-(2-mercapto-tiyazolinyl) cyclo2λ5, 4λ5, 6λ5, triphosphazatriene; N4P3Cl5C4S2H4 (5)] were prepared from the reaction of hexachlorocyclotriphosphazatriene (trimer, N3P3Cl6, 1), with methimazole (2) or MTZ (3) in tetrahydrofurane (THF) in the presence of metallic sodium under an argon atmosphere.
In second step; monospiro2-mercaptothiazolinyl (7a) and dispiro2-mercapto-1-methylimidazolyl sübstituted (6) phosphazene derivatives were obtained from the reactions of 4 and 5 with 2,2′-dihydroxybiphenyl (8) in acetone in the presence of potassium carbonate (K2CO3). Structural characterizations of all obtained compounds were made using by elemental analysis, FT-IR, 1H, 13C and 31P NMR spectroscopies. Compounds 5–7a are reported for the first time in this study.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


