一种用于合成某些苯并咪唑、苯并恶唑和苯并噻唑的高效磁分离 Fe3O4/WO3 催化剂,可用作治疗肾病的潜在药物
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
苯并咪唑衍生物在治疗某些肾脏疾病、肾素抑制剂和调节炎症反应以解决肾病潜在机制方面具有潜力。本文推荐使用从车前子皮还原水提取物中提取的 Fe3O4/WO3 异质结催化剂作为创新催化剂,在温和条件下进行各种苯并咪唑、苯并恶唑和苯并噻唑的一锅合成。通过 XRD、EDS、TEM、FE-SEM、VSM、DRS、XPS 和 FT-IR 等表征方法,可以了解其结构和性质。这种先进的方案在广泛的基质中显示出较高的产率,同时具有环保和高效的特点。此外,该催化剂在五个周期内表现出显著的稳定性,说明其可重复使用而不会影响催化效率。热过滤测试进一步证明了催化剂在反应介质中的稳定性。我们认为,与许多已报道的方案相比,Fe3O4/WO3 催化剂在合成苯并咪唑、苯并恶唑和苯并噻唑方面具有多种优势,包括增强的催化活性可促进所需杂环化合物的高效形成。此外,该催化剂的磁性保证了其高度的可重复使用性,并可通过使用外部磁铁直接进行工作。这一特性有助于在多个合成循环中重复使用,最终减少浪费,降低成本。此外,Fe3O4/WO3 催化剂在温和的条件下工作,最大限度地减少了对苛刻试剂的需求,有助于实现更环保的合成。该方法具有成本效益,催化剂易于回收,为可持续高效合成取代的苯并咪唑、苯并恶唑和苯并噻唑开辟了新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
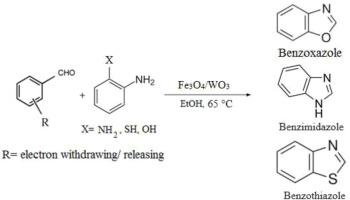
A highly efficient and magnetically separable Fe3O4/WO3 catalyst for the synthesis of some benzimidazoles, benzoxazoles, and benzothiazoles, serving as potential drugs to treat nephropathy diseases
Benzimidazole derivatives have shown potential in treating certain kidney diseases, renin inhibitors, and modulate inflammatory responses to address underlying mechanisms in nephropathy. Herein, Fe3O4/WO3 heterojunction catalyst, derived from the reducing aqueous extract of Plantain peel, is recommended as an innovative catalyst for the one-pot synthesis of various benzimidazoles, benzoxazoles, and benzothiazoles in mild conditions. Through characterization methods like XRD, EDS, TEM, FE-SEM, VSM, DRS, XPS, and FT-IR, its structure and properties are understood. This cutting-edge protocol showcases high yield across a broad range of substrates, all while being eco-friendly and efficient. Moreover, the catalyst exhibited remarkable stability over five cycles, illustrating its reusability without compromising catalytic efficiency. The hot filtration test further underlines its stability in the reaction medium. We believe that Fe3O4/WO3 catalyst offers several advantages in the synthesis of benzimidazoles, benzoxazoles, and benzothiazoles, including enhanced catalytic activity that promotes efficient formation of the desired heterocyclic compounds compared to many reported protocols. Furthermore, its magnetic properties guarantee high reusability and a straightforward work up by using an external magnet. This feature facilitates its reuse in multiple synthesis cycles, ultimately reducing waste and lowering costs. In addition, the Fe3O4/WO3 catalyst operates under mild conditions, minimizing the need for harsh reagents and contributing to a more environmental-friendly synthesis. With cost-effectiveness and easy recovery of the catalyst, this approach sets a new approach for the sustainable and efficient synthesis of substituted benzimidazoles, benzoxazoles, and benzothiazoles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


