一些双(4-甲基-2H-苯并吡喃-2-酮)和多(4-甲基-2H-苯并吡喃-2-酮)衍生物的合成、抗菌、DNA 结合和计算研究
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
研究重点是研究如何通过与相关的双香豆素和多香豆素化合物发生烷基化反应,将 7-hydroxy-4-methyl-2H-chromen-2-one 用作新的双香豆素和多香豆素的构建材料。光谱技术证实了这些化合物的化学成分。实验评估了新香豆素衍生物在受控环境中抑制各种有害细菌菌株生长的效果。在最近形成的化合物中,三香豆素 19 和与噻吩噻吩核 26 连接的双香豆素对受检细菌具有显著的抗菌效果。利用紫外可见光谱评估了化合物 19 和 26 与 CT-DNA 的相互作用,结果发现吸光强度显著降低,并略微向长波长移动。利用计算机模拟分子对接的研究表明,化合物 19 和 26 可以有效地附着在不同的靶蛋白上,如 DNA 回旋酶、二氢蝶酸合成酶、MurE 连接酶和二氢叶酸还原酶,结合分数从 -9.76 到 -6.68 千卡/摩尔不等。这表明我们的衍生物可以阻止这些蛋白质的生成,并显示出显著的抗菌特性。此外,还对化合物 19 进行了药物相似性评估,对化合物 26 进行了 ADMET 特性评估,这表明它们有可能成为进一步药物开发的重要候选化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
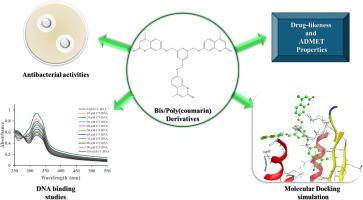
Synthesis, antimicrobial, DNA binding, and computational studies of some bis- and poly(4-methyl-2H-chromen-2-one) derivatives
The research focused on studying how 7-hydroxy-4-methyl-2H-chromen-2-one can be used as a building material for new bis- and poly(coumarins) through alkylation with related bis- and poly(halo) compounds. Spectroscopic techniques confirmed the chemical compositions of these compounds. An experiment was conducted to assess the effectiveness of new coumarin derivatives in inhibiting the growth of various harmful bacterial strains in a controlled environment. Among the recently formed compounds, tris-coumarin 19 and bis-coumarin linked to thienothiophene core 26 demonstrated notable antibacterial effectiveness against the bacteria examined. Compounds 19 and 26 were evaluated for their interactions with CT-DNA using UV-Vis spectroscopy, resulting in a significant decrease in absorbance intensity with a slight shift towards longer wavelengths. Research using computer simulations of molecular docking indicates that compounds 19 and 26 can effectively attach to different target proteins such as DNA gyrase, dihydropteroate synthase, MurE ligase, and dihydrofolate reductase, achieving binding scores ranging from -9.76 to -6.68 kcal/mole. This indicates that our derivatives can prevent these proteins and display significant antibacterial characteristics. Additionally, assessments were done on compound 19 for drug-likeness and compound 26 for ADMET properties, indicating their potential as valuable candidates for further drug development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


