新型二茂铁钙化(Se,Te)苯并咪唑、苯并咪唑鎓盐和钙化酮:结构、电化学、热研究和抗氧化活性
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
介绍了在悬臂中含有有机钙原(Se 和 Te)原子的新二茂铁化苯并咪唑、相应的二茂铁化 NHCs 盐及其硫酮和硒酮衍生物的合成。所有这些化合物都通过红外线(IR)、多核(1H、13C、77Se 和 125Te)一维和二维核磁共振光谱以及质谱法进行了表征。五种化合物的分子结构分别为1-(1-(ferrocenylselenyl)methyl)-benzimidazole (1)、1-(2-(ferrocenylselenyl)ethyl)-benzimidazole (3)、1-(2-(ferrocenyltelluro)ethyl)-benzimidazole (4)、通过 X 射线晶体学测定了 3-二茂铁基甲基-1-(2-(二茂铁基硒)乙基)苯并咪唑碘化物 (8)、3-甲基-1-(2-(二茂铁基硒)乙基)-苯并咪唑啉-2-硒酮 (11)。在这些化合物中,CH⋯N、CH⋯C、CH⋯S、CH⋯Se、CH⋯I、CH⋯O 和 OH⋯I 等多种分子间氢键相互作用促成了超分子结构的形成。此外,还对这些二茂铁瑀化苯并咪唑衍生物进行了循环伏安(CV)和热重(TGA)研究。所有化合物(1-7)的循环伏安图都在较高的正电位下显示出一个明确的二茂铁波,而硒化苯并咪唑(1)和(3)及其苯并咪唑鎓盐(5)和(6)则显示出另一个不可逆的单电子氧化还原波。钙化苯并咪唑盐的 TGA 曲线显示,化合物(6)和(7)的分解步骤为三步,化合物(8)的分解步骤为四步。苯并咪唑前体(1-4)和苯并咪唑鎓盐(5-7)的抗氧化活性已通过硫代巴比妥酸活性物质(TBARS)检测进行了评估。确定了所有化合物的 IC50 值。此外,还研究了所有这些化合物在三种不同浓度(1、10 和 100 μM)下对盐蒿模型的毒性试验。本文章由计算机程序翻译,如有差异,请以英文原文为准。
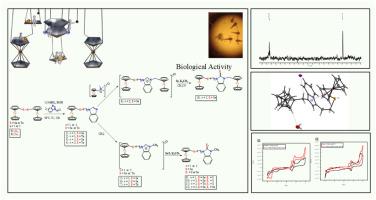
New ferrocenyl chalcogenated (Se, Te) benzimidazoles, benzimidazolium salts and chalcogenones: Structure, electrochemistry, thermal investigation and antioxidant activity
Synthesis of new ferrocenylated benzimidazoles containing organochalcogen donor (Se and Te) atoms in the pendant arm, their corresponding ferrocenylated-NHCs salts and their thione and selenone derivatives have been described. All these compounds were characterized by Infrared (IR), multinuclear (1H, 13C, 77Se and 125Te) 1D and 2D NMR spectroscopy and mass spectrometry. The molecular structures of five compounds, viz. 1-(1-(ferrocenylselenyl)methyl)-benzimidazole (1), 1-(2-(ferrocenylselenyl)ethyl)-benzimidazole (3), 1-(2-(ferrocenyltelluro)ethyl)-benzimidazole (4), 3-ferrocenyl methyl-1-(2-(ferrocenylselenyl)ethyl)benzimidazolium iodide (8) 3-methyl-1-(2-(ferrocenylselenyl)ethyl)-benzimidazolin-2-selenone (11) were determined by X-ray crystallography. In these compounds, multiple intermolecular hydrogen bonding interactions, CH⋯N, CH⋯C, CH⋯S, CH⋯Se, CH⋯I, CH⋯O, and OH⋯I contribute to the formation of supramolecular structures. Cyclic voltammetric (CV) and thermogravimetric (TGA) studies of these ferrocenyl chalcogenated benzimidazole derivatives have also been carried out. The cyclic voltammogram for all the compounds (1-7) shows one well-defined ferrocene-based wave at a higher positive potential while in the selenated benzimidazoles (1) and (3) and their benzimidazolium salts (5) and (6) present another irreversible single-electron redox wave. The TGA curves of chalcogenated benzimidazolium salts show three steps of decomposition for compounds (6) and (7), and four steps of decomposition for compound (8). The antioxidant activity of benzimidazole precursors (1-4) and benzimidazolium salts (5-7) have been evaluated by conducting a thiobarbituric acid reactive substance (TBARS) assay. The IC50 value of all the compounds has been determined. The toxicity assay of all these compounds against Artemia Salina model at three different concentrations (1, 10 and 100 μM) was also studied.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


