瘦素受体纤连蛋白 III 型结构域中的致病变体:分子动力学模拟和结构分析
IF 2.7
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
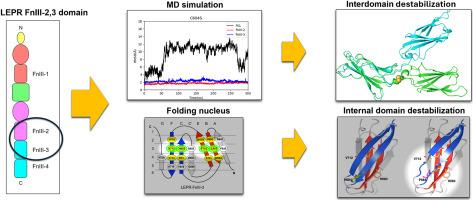
一些病例报告发现瘦素受体(LEPR)变体与人类严重肥胖有关。然而,直到最近,人们才对瘦素受体的结构有了部分了解,很少有研究调查这些变体对蛋白质三维结构的不利影响。值得注意的是,纤连蛋白 III 型(FnIII)结构域在信号转导中起着至关重要的作用。在这项研究中,我们利用分子动力学(MD)模拟和结构分析研究了 FnIII 结构域中的 10 个变体对 LEPR 结构的影响。我们的 300 ns MD 模拟显示,C604S 变体破坏了一个关键的二硫键,显著增加了 FnIII-2 和 FnIII-3 结构域的整体均方根偏差(RMSD),表明正常信号传导所需的结构域间刚性失稳。P639L、N718S和W646C等变异也诱导了FnIII结构域之间的异常弯曲和旋转错位,导致了结构域间的不稳定。结构分析确定了折叠核,并证明 L662S、W664R、H684P 和 S723F 会破坏内部结构域的稳定性。影响结构域间的变异导致生物信息学工具的损伤预测得分低于预期。这项研究有望为阐明瘦素受体错义变异的致病机制做出贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pathogenic variants in the fibronectin type III domain of leptin receptor: Molecular dynamics simulation and structural analysis
Several case reports have identified leptin receptor (LEPR) variants associated with severe obesity in humans. However, the structure of LEPR has only been partially understood until recently, and few studies have investigated the detrimental effects of these variants on the protein's three-dimensional structure. Notably, fibronectin type III (FnIII) domains play a crucial role in signal transduction. In this study, we examined the impact of 10 variants within the FnIII domains on LEPR structure using molecular dynamics (MD) simulations and structural analysis. Our 300 ns MD simulations revealed that the C604S variant, which disrupts a key disulfide bond, significantly increased the overall root-mean-square deviation (RMSD) of the FnIII-2 and FnIII-3 domains, indicating destabilization of the interdomain rigidity required for proper signaling. Variants such as P639L, N718S, and W646C also induced abnormal bending and rotational misalignment between the FnIII domains, contributing to interdomain destabilization. Structural analysis identified folding nuclei and demonstrated that L662S, W664R, H684P, and S723F destabilize the internal domain. Variants affecting interdomain resulted in lower-than-expected damage prediction scores by bioinformatics tools. This study is expected to contribute to the elucidation of the disease-causing mechanisms of missense variants in the leptin receptor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of molecular graphics & modelling
生物-计算机:跨学科应用
CiteScore
5.50
自引率
6.90%
发文量
216
审稿时长
35 days
期刊介绍:
The Journal of Molecular Graphics and Modelling is devoted to the publication of papers on the uses of computers in theoretical investigations of molecular structure, function, interaction, and design. The scope of the journal includes all aspects of molecular modeling and computational chemistry, including, for instance, the study of molecular shape and properties, molecular simulations, protein and polymer engineering, drug design, materials design, structure-activity and structure-property relationships, database mining, and compound library design.
As a primary research journal, JMGM seeks to bring new knowledge to the attention of our readers. As such, submissions to the journal need to not only report results, but must draw conclusions and explore implications of the work presented. Authors are strongly encouraged to bear this in mind when preparing manuscripts. Routine applications of standard modelling approaches, providing only very limited new scientific insight, will not meet our criteria for publication. Reproducibility of reported calculations is an important issue. Wherever possible, we urge authors to enhance their papers with Supplementary Data, for example, in QSAR studies machine-readable versions of molecular datasets or in the development of new force-field parameters versions of the topology and force field parameter files. Routine applications of existing methods that do not lead to genuinely new insight will not be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


