免疫原性清除与 PD-1 阻断相结合,通过促进效应记忆型 CD8+ T 细胞的招募和扩增而产生抗肿瘤效果
IF 5
2区 医学
Q2 Medicine
引用次数: 0
摘要
免疫检查点抑制剂有望用于癌症治疗,但只有少数患者对此有反应。为了克服这种抗药性,人们探索了联合策略。将使用免疫原性细胞死亡诱导剂的免疫原性清除与 rho 激酶抑制剂相结合,可增强抗原递呈细胞对免疫原性死亡癌细胞的吞噬作用,通过树突状细胞介导的引物激活 CD8+ T 细胞,从而刺激肿瘤特异性免疫反应。这种方法提高了免疫检查点阻断(ICB)耐受性癌症对 ICB 的反应性。然而,其确切机制仍不清楚。本研究阐明了免疫原清除增强 ICB 反应的细胞机制。通过单细胞 RNA 测序,我们观察到肿瘤微环境中的效应记忆类 CD8+ T 细胞在联合治疗后有所增加。我们认为,这种细胞群可能来自于免疫原清除后增殖的 CD8+ T 细胞。值得注意的是,ICB 反应患者体内大量的效应记忆样 CD8+ T 细胞表明它们具有抗肿瘤作用。因此,通过增强 T 细胞引物来增加这种细胞群,可能会改善对 ICB 耐药的肿瘤的反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
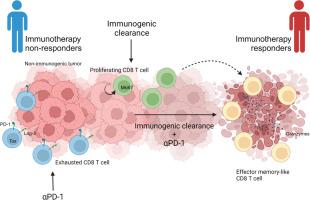
Immunogenic clearance combined with PD-1 blockade elicits antitumor effect by promoting the recruitment and expansion of the effector memory-like CD8+ T cell
Immune checkpoint inhibition shows promise for cancer treatment, but only a minority of patients respond. Combination strategies have been explored to overcome this resistance. Combining immunogenic clearance using immunogenic cell death inducers with a rho kinase inhibitor enhances phagocytosis of immunogenically dying cancer cells by antigen-presenting cells, stimulating tumor-specific immune responses by activating CD8+ T cells via dendritic cell-mediated priming. This approach increases the responsiveness of immune checkpoint blockade (ICB)-resistant cancer to ICB. However, the precise mechanisms remain unclear. This study elucidates cellular mechanisms of immunogenic clearance enhancing ICB response. Using single-cell RNA sequencing, we observed an increase in effector memory-like CD8+ T cells within the tumor microenvironment with combined treatment. We propose this cell cluster may originate from proliferating CD8+ T cells elevated by immunogenic clearance. Notably, abundant effector memory-like CD8+ T cells in ICB-responsive patients suggest their antitumor effect. Thus, increasing this cell population through enhanced T cell priming may improve the response of ICB-resistant tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


