大麻二酚的立体异构体及其药理活性--大麻素的潜在新方向
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
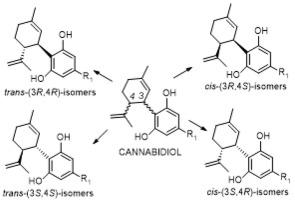
大麻二酚(CBD)是一种由大麻属植物生物合成的双环非精神活性大麻素,因其治疗特性在过去十年中引起了人们的极大兴趣。2018 年,美国 FDA 批准了一种基于 CBD 的药物 Epidiolex®,用于治疗两种罕见的癫痫发作性疾病。CBD 在其萜类分子的 C3 和 C4 上具有两个手性中心,并沿着 C3-C4 键轴呈现顺反立体异构。(-)-反式-(3R,4R)-CBD(天然 CBD)由大麻植物生物合成,而非天然的(+)-反式-(3S,4S)-CBD 则通过化学合成获得。这两种反式异构体都具有广泛的体外和体内生物活性;与相应的(-)-反式异构体相比,非天然立体异构体(+)-反式-CBD及其衍生物通常具有更强的活性。另一方面,顺式-CBD异构体最近才有报道,而且会发生外嵌合反应变成反式异构体。探索CBD立体异构体的独特合成方法和生物活性是一个重要机会,这可能为开发新型疗法铺平道路。在这里,作为大麻素的一个新方向,我们回顾了 CBD 立体异构体的化学性质、它们的结构-活性关系、靶点选择性和在动物模型中的疗效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Stereoisomers of cannabidiols and their pharmacological activities – A potentially novel direction for cannabinoids
Cannabidiol (CBD), a bicyclic non-psychoactive cannabinoid biosynthesized by Cannabis spp. of plants, has attracted significant interest in the past decade due to its therapeutic properties. In 2018, the US FDA approved Epidiolex®, a CBD-based drug for the treatment of two rare epileptic seizure disorders.
CBD possesses two chiral centers at C3 and C4 on its terpenoid moiety and exhibits cis–trans stereoisomerism along the C3–C4 bond axis. (−)-trans-(3R,4R)-CBD, the natural CBD, is biosynthesized by the cannabis plant, while the unnatural (+)-trans-(3S,4S)-CBD is obtained via chemical synthesis. Both trans isomers exhibit broad in vitro and in vivo biological activities; typically, the unnatural stereoisomer (+)-trans-CBD and its derivatives exhibited more potent activities in comparison to the corresponding (−)-trans isomers. On the other hand, cis-CBD isomers have only been reported recently and can undergo epimerization into trans isomers.
There is a significant opportunity to explore unique synthetic methods and biological activities of stereoisomers of CBD that may pave the path for the development of novel therapeutics. Herein, as a novel direction in cannabinoids, we review the chemistry of CBD stereoisomers, their structure–activity relationships, target selectivity and efficacy in animal models.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


