发现具有神经保护作用的强效选择性 TRPC3 拮抗剂
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
TRPC3 蛋白在钙信号转导中发挥着关键作用,影响着细胞功能。TRPC3 的异常表达与心血管疾病、肿瘤和神经变性等多种病症有关。尽管 TRPC3 与 TRPC6 和 TRPC7 在功能上相似,但在疾病中却表现出不同的作用。因此,开发一种具有药物特性的强效、选择性 TRPC3 拮抗剂至关重要。我们采用了大量的药物化学合成和结构-活性关系(SARs)研究。以先导化合物 JW-65 为支架,设计并合成了 31 种新型 TRPC3 拮抗剂。化合物 60a 的药效提高了 4 倍,并显示出卓越的选择性。该化合物具有良好的类药物特性,体外神经元保护效果更强。分子建模显示了 TRPC3 蛋白与其拮抗剂之间可能的作用模式。总之,60a 在与 TRPC3 失调有关的疾病的临床开发方面前景广阔。本文章由计算机程序翻译,如有差异,请以英文原文为准。
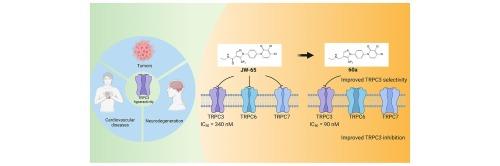
Discovery of a potent and selective TRPC3 antagonist with neuroprotective effects
The TRPC3 protein plays a pivotal role in calcium signaling, influencing cell function. Aberrant TRPC3 expression is implicated in various pathologies, including cardiovascular diseases, tumors, and neurodegeneration. Despite its functional similarities with TRPC6 and TRPC7, TRPC3 exhibits distinct roles in disease contexts. Therefore, it is of paramount importance to develop a potent and selective TRPC3 antagonist with favorable drug-like properties. We employed extensive medicinal chemistry synthesis and structure–activity relationships (SARs) study. Thirty-one novel TRPC3 antagonists were designed and synthesized using the lead compound JW-65 as the scaffold. Compound 60a exhibits a 4-fold improvement in potency and displays exceptional selectivity. With favorable drug-like properties, this compound shows a heightened in vitro neuronal protective effect. Molecular modeling suggests possible modes of action between the TRPC3 protein and its antagonists. In summary, 60a holds significant promise for clinical development in conditions associated with TRPC3 dysregulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


