在钴催化下利用 N-氯酰胺的双重反应活性进行吲哚的级联 C-H 氧化/氯化反应:推翻霍夫曼重排途径,实现氨基羰基化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们利用 N-氯酰胺在常温下的双重功能性,开发了一种 Cp*Co(III)- 催化的吲哚级联 C-2 氨化/C-3 氯化反应。该方案避免了通过 N-氯酰胺的潜在霍夫曼重排形成的异氰酸酯的 C-H 功能化可能导致的氨基羰基化途径。事实上,这是首个使用 N-氯酰胺作为酰胺化剂进行定向 C-H 酰胺化的实例。对照实验表明,C-2 C-H 氨化发生在 C-3 氯化之前。此外,氯功能还被有效地用于构建 C-S 和 C-N 键,从而扩大了合成化合物的分子多样性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
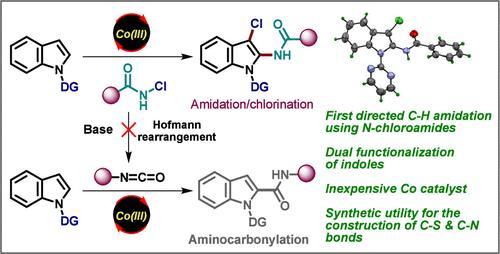
Harnessing Dual Reactivity of N-Chloroamides for Cascade C–H Amidation/Chlorination of Indoles under Cobalt-Catalysis: Overriding Hofmann Rearrangement Pathway Leading to Aminocarbonylation
Herein, we have developed a Cp*Co(III)-catalyzed cascade C-2 amidation/C-3 chlorination of indoles by leveraging the dual functionality of N-chloroamides at ambient temperature. This protocol avoids the aminocarbonylation pathway that may result from the C–H functionalization of isocyanates formed via a potential Hofmann rearrangement of N-chloroamides. In fact, this represents the first example of directed C–H amidation using N-chloroamides as amidating agent. The control experiment indicated that the C-2 C–H amidation occurs prior to C-3 chlorination. Additionally, chloro functionality has been effectively utilized for the construction of C–S and C–N bonds, thereby expanding the molecular diversity of the synthesized compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


