混合氨基酸 PET 深度相互学习预测中线胶质瘤中的 H3K27M 突变
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
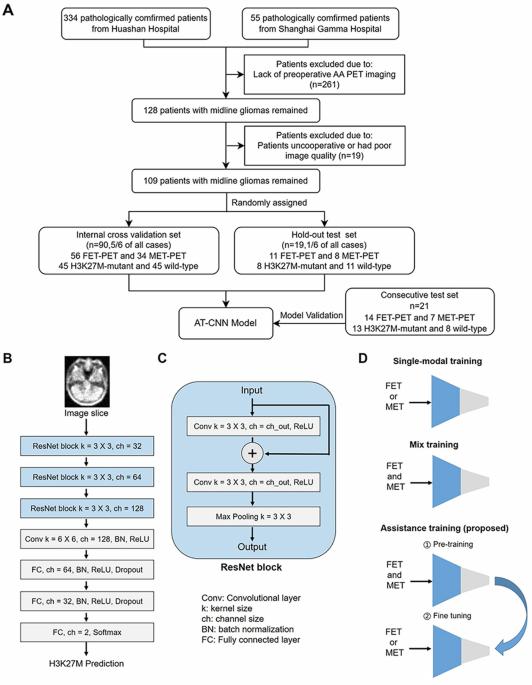
无创预测中线胶质瘤的H3K27M突变状态引起了广泛关注,特别是利用11C-蛋氨酸(MET)和18F-氟乙基酪氨酸(FET)正电子发射断层扫描(PET)进行深度学习。为了优化预测效率,我们推导出一种辅助训练(AT)方案,使 MET 和 FET 学习互惠互利,从而提高预测能力,但仍只需要其中一种 PET 作为预测输入。我们的方法大大超过了传统的卷积神经网络(CNN)、基于放射组学和基于 MR 的方法,在内部交叉验证(n = 90)中,MET 的曲线下面积(AUC)达到了 0.9343,FET 的曲线下面积(AUC)达到了 0.8619。在保留测试(n = 19)和连续测试群组(n = 21)中,其性能仍然很高,AUC 分别为 0.9205 和 0.7404。在病理不确定的病例中,多部门决策和结果的一致性证实了所提方法的临床可行性。这些研究结果将我们的方法定位为中线胶质瘤治疗决策的辅助工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Deep mutual learning on hybrid amino acid PET predicts H3K27M mutations in midline gliomas
Predicting H3K27M mutation status in midline gliomas non-invasively is of considerable interest, particularly using deep learning with 11C-methionine (MET) and 18F-fluoroethyltyrosine (FET) positron emission tomography (PET). To optimise prediction efficiency, we derived an assistance training (AT) scheme to allow mutual benefits between MET and FET learning to boost the predictability but still only require either PET as inputs for predictions. Our method significantly surpassed conventional convolutional neural network (CNN), radiomics-based, and MR-based methods, achieved an area under the curve (AUC) of 0.9343 for MET, and an AUC of 0.8619 for FET during internal cross-validation (n = 90). The performance remained high in hold-out testing (n = 19) and consecutive testing cohorts (n = 21), with AUCs of 0.9205 and 0.7404. The clinical feasibility of the proposed method was confirmed by the agreements to multi-departmental decisions and outcomes in pathology-uncertain cases. The findings positions our method as a promising tool for aiding treatment decisions in midline glioma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


