血红蛋白α是T淋巴细胞中对氧化还原反应敏感的线粒体相关蛋白。
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
血红蛋白亚基是一种特征明确的四聚体载氧蛋白,最近有研究表明,血红蛋白亚基在各种非典型细胞类型中都有表达。然而,血红蛋白亚基在这些细胞中的确切功能仍有待全面阐明。在此,我们首次报道了血红蛋白α-a1(Hba-a1)在T淋巴细胞中的表达,并描述了它作为线粒体相关抗氧化剂的作用。在幼稚 T 淋巴细胞中,Hba-a1 mRNA 和 HBA 蛋白存在,并在氧化还原扰动(尤其是线粒体产生的扰动)的作用下被高度诱导。此外,使用 T 淋巴细胞特异性 Hba-a1 基因敲除小鼠模型的初步数据表明,Hba-a1 的缺失会导致线粒体活性氧和炎症细胞因子在应激挑战后加速产生,进一步支持了 HBA 缓冲线粒体氧化还原环境的作用。有趣的是,我们观察到 Hba-a1 的表达随 T 淋巴细胞极化和代谢状态的不同而显著上调或下调,这似乎是由转录调控和染色质重塑共同控制的。总之,这些数据表明,Hba-a1 可能是一种关键的线粒体相关抗氧化剂,而且似乎具有与 T 淋巴细胞活化和分化相关的关键而复杂的功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
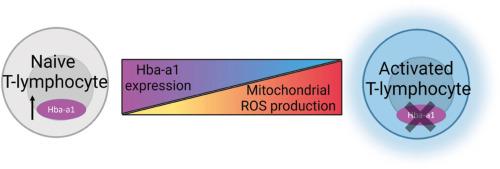
Hemoglobin alpha is a redox-sensitive mitochondrial-related protein in T-lymphocytes
Hemoglobin subunits, which form the well-characterized, tetrameric, oxygen-carrying protein, have recently been described to be expressed in various non-canonical cell types. However, the exact function of hemoglobin subunits within these cells remains to be fully elucidated. Herein, we report for the first time, the expression of hemoglobin alpha-a1 (Hba-a1) in T-lymphocytes and describe its role as a mitochondrial-associated antioxidant. Within naïve T-lymphocytes, Hba-a1 mRNA and HBA protein are present and highly induced by redox perturbations, particularly those arising from the mitochondria. Additionally, preliminary data using a T-lymphocyte specific Hba-a1 knock-out mouse model indicated that the loss of Hba-a1 led to an exacerbated production of mitochondrial reactive oxygen species and inflammatory cytokines after a stress challenge, further supporting the role of HBA acting to buffer the mitochondrial redox environment. Interestingly, we observed Hba-a1 expression to be significantly upregulated or downregulated depending on T-lymphocyte polarization and metabolic state, which appeared to be controlled by both transcriptional regulation and chromatin remodeling. Altogether, these data suggest Hba-a1 may function as a crucial mitochondrial-associated antioxidant and appears to possess critical and complex functions related to T-lymphocyte activation and differentiation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


