从一维 CsPbBr3 Perovskite 纳米线的本征手性构件中获得奇光特性
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
手性在生物系统中无处不在,甚至在某些无机晶体中也会出现。有趣的是,一些无机纳米晶体尽管是非手性体态,但也被证明具有手性。然而,由于用于钝化此类纳米晶体的手性有机配体的存在,此类纳米晶体的手性形成机制和自旋响应仍然模糊不清。在这里,我们从不同长度尺度的卤化铅过氧化物纳米线中认识到了其内在的自旋响应。立方体连接的纳米线具有最小的界面接触,即使在没有手性配体的情况下,它们的排列也具有手性,从而产生了气光响应。不同长度的手性纳米线可作为一个系统平台,随着长度的增加,不对称因子也会显著提高。最长纳米线的不对称因子达到 1.4 × 10-2,是目前本征手性包晶纳米晶体中最高的。纳米线产生圆极化发光,这在没有任何手性配体的卤化物包晶纳米晶体中鲜有报道。此外,我们还发现,手性存在于由两个角连接立方体组成的二聚体形式的基本单元中。纳米线的内在手性是由相连立方体沿界面边界的晶格旋转决定的,这与通常观察到的手性配体诱导的手性不同。这种手性卤化铅包光体纳米晶体具有强大的手性特性,为了解本征手性的起源和合理设计各向异性手性纳米结构提供了一个理想的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
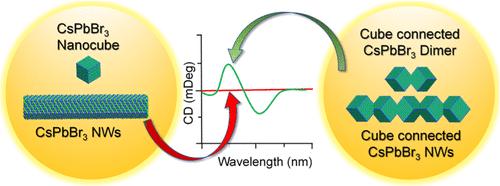
Deriving Chiroptical Properties from Intrinsically Achiral Building Blocks of One-Dimensional CsPbBr3 Perovskite Nanowires
Chirality is a ubiquitous feature in biological systems and occurs even in certain inorganic crystals. Interestingly, some inorganic nanocrystals have been shown to possess chirality, despite their achiral bulk forms. However, the mechanism of chirality formation and chiroptical responses in such nanocrystals is still ambiguous due to the presence of chiral organic ligands used to passivate such nanocrystals. Here, we recognize intrinsic chiroptical responses from lead halide perovskite nanowires with different length scales. Cube-connected nanowires with minimum interfacial contacts make their arrangement chiral for chiroptical responses even in the absence of chiral ligands. The chiral nanowires with varying lengths serve as a systematic platform for improving dissymmetric factors significantly with increasing lengths. The dissymmetric factor of the longest nanowires reaches 1.4 × 10–2, which is the highest among the intrinsic chiral perovskite nanocrystals at present. The nanowires generate circularly polarized luminescence, which has been seldom reported in halide perovskite nanocrystals in the absence of any chiral ligands. Furthermore, we find that chirality exists in the basic unit consisting of two corner-connected cubes in the form of a dimer. The intrinsic chirality of the nanowires is determined by the lattice rotation of connected cubes along the interfacial boundaries, which is different from the commonly observed chirality induced by chiral ligands. Such chiral lead halide perovskite nanocrystals with robust chiroptical properties provide an ideal platform for understanding the origin of intrinsic chirality and the rational design of anisotropic chiral nanostructures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


