通过 2H-Imidazoles 与 CF3-Ynones 的级联反应合成 CF3-Azafluorenes
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报告了一种基于 5-芳基-2H-咪唑与 CF3-炔酮反应的三氟甲基 (CF3) 取代氮杂芴的简易合成方法。该反应通过 C-H 活化引发的正式 [3+2] 环加成反应,得到作为关键中间体的螺[咪唑-4,1′-茚],然后进行其逆向 [3+2] 环加成、异构化和烯胺亲核加成。这种通过级联 C-H/C-C/C-N 键裂解和 C≡C 键形成同时形成茚和吡啶支架来合成 CF3-氮杂芴衍生物的方法以前从未公开过。此外,由此获得的产品对癌细胞株具有抗增殖活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
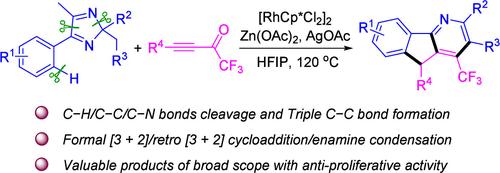
Synthesis of CF3-Azafluorenes through the Cascade Reaction of 2H-Imidazoles with CF3-Ynones
A concise synthesis of trifluoromethyl (CF3)-substituted azafluorenes based on the reaction of 5-aryl-2H-imidazoles with CF3-ynones is reported. The reaction proceeds through C–H activation-initiated formal [3+2] cycloaddition to give spiro[imidazole-4,1′-indene] as a key intermediate, followed by its retro [3+2] cycloaddition, isomerization, and enamine nucleophilic addition. This synthesis of CF3-azafluorene derivatives via simultaneous formation of both the indene and the pyridine scaffolds through cascade C–H/C–C/C–N bond cleavage and C≡C bond formation has not been disclosed before. Moreover, the products thus obtained showed antiproliferative activity against cancer cell lines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


