Co3Mn-CO3 LDH 的运算 X 射线吸收光谱:电化学条件下的形成和结构演变
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
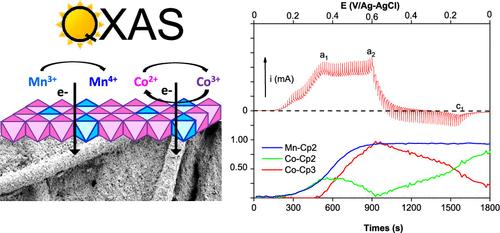
本文旨在阐明 CoMn 层状双氢氧化物(LDH)的形成机制,并研究钴和锰这两种金属阳离子在这种片状化合物的电化学行为中的作用。在时间分辨同步辐射 X 射线吸收光谱(QXAS)数据中应用了交替最小二乘法多变量曲线分辨率(MCR-ALS)分析法,以监测 Co3Mn LDH 形成和电化学过程中 Co 和 Mn 价态的标示情况。钴和锰 K 边的 QXAS 实验记录了合成过程的三个步骤:(i) 快速共沉淀和两个连续的老化步骤 (ii) 在氩气中和 (iii) 在空气中。在与快速共沉淀相对应的第一步中,类似青金石的 Co/Mn(OH)2 混合相迅速沉淀,在前两个步骤((i) 和 (ii))中没有发生金属阳离子的氧化。在空气环境下的最后一个干燥步骤(iii)中,Mn(II)被氧气拓扑氧化成 Mn(III),并在碳酸盐的插层作用下形成 Co(II)3Mn(III)-CO3 LDH。最后,强调了金属阳离子 Co(II) 和 Mn(III) 在电化学过程中的氧化还原作用,并提出了 Co 位点之间的电子跳跃机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Operando X-ray Absorption Spectroscopy of Co3Mn–CO3 LDH: Formation and Structural Evolution under Electrochemical Conditions
The purpose of this article is to elucidate the mechanism of formation of CoMn layered double hydroxide (LDH) and to study the role of both metal cations, cobalt and manganese, in the electrochemical behavior of this lamellar compound. Multivariate curve resolution with alternating least squares (MCR-ALS) analysis was applied to time-resolved synchrotron X-ray absorption spectroscopy (QXAS) data, monitoring the speciation of Co and Mn valence states under both Co3Mn LDH formation and electrochemical processes. QXAS experiments at Co and Mn K-edges were recorded all over three steps of the synthesis process: (i) fast coprecipitation and two successive aging steps (ii) under Ar and (iii) in air. In the first step, corresponding to the fast coprecipitation, a brucite-like Co/Mn(OH)2 mixed phase precipitates rapidly and no oxidation of metal cations occurs during the two first steps ((i) and (ii)). Topotactic oxidation of Mn(II) into Mn(III) by oxygen occurs during the final drying step under an air atmosphere (iii), assisted by the intercalation of carbonate, leading then to the formation of Co(II)3Mn(III)–CO3 LDH. Finally, the redox contribution of both metal cations Co(II) and Mn(III) in the electrochemical process is highlighted, and a mechanism of electron hopping between Co sites is proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


