MOTS-c通过激活MEF2A缓解肝细胞癌在缺氧条件下对TRAIL诱导的细胞凋亡的抵抗。
IF 3.3
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
背景:12S rRNA-c型线粒体ORF(MOTS-c)作为一种AMPK激动剂,可以调节适应性核基因的表达,从而促进细胞稳态。然而,有关 MOTS-c 在肝细胞癌(HCC)中的作用的研究尚不充分。本研究旨在揭示 MOTS-c 对 HCC 细胞凋亡的作用。方法:Huh7 和 HCCLM3 细胞在缺氧条件下与 MOTS-c 共同孵育,用酶联免疫吸附试验定量检测 HCC 患者和健康对照组外周血中 MOTS-c 的水平。细胞活力通过 3-(4,5-二甲基thazol-2-yl)-2,5-二苯基溴化四氮唑(MTT)检测。细胞凋亡通过流式细胞术和 Tunel 试验进行检测。蛋白表达通过 Western 印迹或免疫组化检测。通过双荧光素酶报告实验和染色质免疫沉淀实验确定了肌细胞增强因子 2A(MEF2A)、死亡受体 4(DR4)和 DR5 之间的关联。通过肿瘤裸鼠模型评估MOTS-c对体内HCC肿瘤形成的影响:结果:HCC患者外周血中的MOTS-c水平明显低于健康人。在缺氧条件下,MOTS-c能促进HCC细胞凋亡。缺氧刺激会降低MEF2A、DR4、DR5、fas-associating via death domain(FADD)和caspase-8的蛋白表达,而MOTS-c处理后这些效应会减弱。在缺氧处理条件下,MOTS-c通过磷酸化AMPK激活MEF2A,从而诱导TRAIL诱导的HCC细胞凋亡。此外,MEF2A还能转录上调DR4和DR5。MOTS-c 激活了 MEF2A,从而调节 DR4 和 DR5 的表达,进一步介导 TRAIL 诱导的细胞凋亡。此外,MOTS-c还能缓解缺氧诱导的体内肿瘤生长:结论:MOTS-c通过激活MEF2A缓解了缺氧诱导的HCC细胞对TRAIL诱导的凋亡的抵抗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
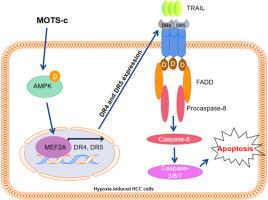
MOTS-c relieves hepatocellular carcinoma resistance to TRAIL-induced apoptosis under hypoxic conditions by activating MEF2A
Background
Mitochondrial ORF of the 12S rRNA type-c (MOTS-c) as an AMPK agonist can regulate the expression of adaptive nuclear genes to promote cell homeostasis. However, the investigation of MOTS-c in hepatocellular carcinoma (HCC) is insufficient. This study aims to reveal the role of MOTS-c on HCC cell apoptosis.
Methods
Huh7 and HCCLM3 cells were incubated with MOTS-c under a hypoxic condition. MOTS-c levels were quantified by enzyme-linked immunosorbent assay in the peripheral blood of HCC patients and healthy controls. Cell viability was detected by 3-(4,5-Dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cell apoptosis was investigated by flow cytometry and Tunel assay. Protein expression was detected by western blotting or immunohistochemistry assay. Dual-luciferase reporter assay and chromatin immunoprecipitation assay were performed to identify the association among myocyte enhancer factor 2A (MEF2A), death receptor 4 (DR4) and DR5. A tumor-bearing nude mouse model was conducted to assess the effect of MOTS-c on HCC tumor formation in vivo.
Results
MOTS-c levels in the peripheral blood of HCC patients were significantly lower compared to healthy individuals. MOTS-c promoted HCC cell apoptosis under hypoxia conditions. Hypoxia stimulation decreased the protein expression of MEF2A, DR4, DR5, fas-associating via death domain (FADD) and caspase-8, while these effects were attenuated after MOTS-c treatment. MOTS-c induced TRAIL-induced apoptosis of HCC cells by activating MEF2A through the phosphorylation of AMPK under hypoxia treatment. In addition, MEF2A transcriptionally up-regulated DR4 and DR5. MOTS-c activated MEF2A to regulate DR4 and DR5 expression, further mediating TRAIL-induced apoptosis. Further, MOTS-c treatment relieved hypoxia-induced tumor growth in vivo.
Conclusion
MOTS-c relieved hypoxia-induced HCC cell resistance to TRAIL-caused apoptosis by activating MEF2A.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


