封面专题:甲基和相关基团之间的吸引力与排斥力:(CH3NHCH3)2 和 (CH3SeBr2CH3)2 (ChemPhysChem 22/2024)
IF 2.3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们可以在剑桥结构数据库(CSD)中找到许多同源二聚体结构,根据 C⋅⋅C 距离和成键角度标准,我们可能会认为这些结构中存在甲基⋅⋅甲基相互作用。此外,QTAIM、NCI 和 NBO 分析的结果可能会错误地认为它们之间的相互作用是吸引性的,而在某些情况下实际上是排斥性的,相互作用能的正值就是证明。更多信息,请参阅 M. Michalczyk 及其合作者的研究文章(DOI: 10.1002/cphc.202400495)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
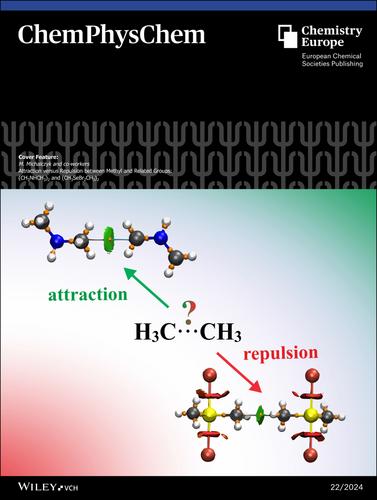
Cover Feature: Attraction versus Repulsion between Methyl and Related Groups: (CH3NHCH3)2 and (CH3SeBr2CH3)2 (ChemPhysChem 22/2024)
One can find numerous homodimeric structures in the Cambridge Structure Database (CSD), where one might assume that there is a methyl⋅⋅⋅methyl interaction in terms of C⋅⋅⋅C distance and bonding angle criteria. Furthermore, the results of QTAIM, NCI and NBO analyses can erroneously suggest that the interaction between them is attractive, whereas in some cases it is actually repulsive, as evidenced by the positive value of the interaction energy. More information can be found in the Research Article by M. Michalczyk and co-workers (DOI: 10.1002/cphc.202400495).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemphyschem
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
3.40%
发文量
425
审稿时长
1.1 months
期刊介绍:
ChemPhysChem is one of the leading chemistry/physics interdisciplinary journals (ISI Impact Factor 2018: 3.077) for physical chemistry and chemical physics. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
ChemPhysChem is an international source for important primary and critical secondary information across the whole field of physical chemistry and chemical physics. It integrates this wide and flourishing field ranging from Solid State and Soft-Matter Research, Electro- and Photochemistry, Femtochemistry and Nanotechnology, Complex Systems, Single-Molecule Research, Clusters and Colloids, Catalysis and Surface Science, Biophysics and Physical Biochemistry, Atmospheric and Environmental Chemistry, and many more topics. ChemPhysChem is peer-reviewed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


