烷基蔗糖酯与 Brijs:链长和温度如何影响表面和泡沫特性
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
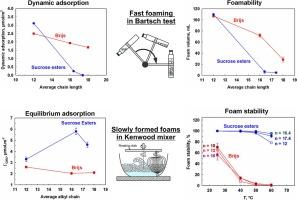
本研究的主要目的是确定单酯含量高(≥ 70%)的烷基蔗糖酯(SE)和头基中乙氧基基团数多(≥ 20)的聚氧乙烯烷基醚(Brijs)在表面、薄膜和泡沫特性方面的异同。实验使用了烷基链长为 12、16 和 18 个碳原子的表面活性剂分子,浓度为 0.01 至 1 wt%,温度范围为 25 °C 至 60 °C。然而,将链长从 12 个碳原子增加到 18 个碳原子后,Brijs 的滞后时间增加了 10 倍,而 SE 的滞后时间增加了 600 多倍。这种效应使长链 SE 无法在 Bartsch 试验中形成大量泡沫,并导致泡沫上升法中气泡与喷射器分离后气泡之间的明显凝聚,从而产生气泡非常大的泡沫,表现出较低的稳定性。使用 Kenwood 混合器产生泡沫时,较长链的 SE 分子有足够的时间吸附在气泡表面,从而产生具有小气泡的大量泡沫,这些泡沫即使在 60 °C 下也能保持稳定。相比之下,由 Brijs 溶液产生的泡沫在 60 °C 下非常不稳定。SEs 泡沫的长期稳定性归因于在气泡表面形成了单酯和二酯混合吸附层。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Alkyl sucrose esters vs. Brijs: How chain length and temperature impact surface and foam properties
The primary objective of this study is to determine the similarities and differences in the surface, film, and foam properties of alkyl sucrose esters (SEs) with high monoester content (≥ 70 %) and polyoxyethylene alkyl ethers (Brijs) with a high number of ethoxy groups (≥ 20) in their head group. Experiments were conducted using surfactant molecules with alkyl chain lengths of 12, 16, and 18 carbon atoms at concentrations between 0.01 and 1 wt%, within a temperature range of 25 °C to 60 °C.
The lag time for surfactant adsorption increased with surfactant chain length, decreased with temperature, and significantly decreased with surfactant concentration for both types of studied surfactants. However, increasing the chain length from 12 to 18 carbon atoms led to a 10-fold increase in lag time for Brijs and more than a 600-fold increase for SEs. This effect rendered longer-chain SEs incapable of forming voluminous foam in Bartsch test and led to pronounced coalescence between the bubbles after their separation from the sparger in the foam rise method, resulting in foams with very large bubbles, which exhibited lower stability. The utilization of a Kenwood mixer for foam generation provided sufficient time for longer-chain SE molecules to adsorb on the bubble surfaces and to produce voluminous foams with small bubbles, which remained stable even at 60 °C. In contrast, foams generated from Brijs solutions are very unstable at 60 °C. The long-standing stability of SEs foam was attributed to the formation of mixed mono- and diesters adsorption layers on the bubble surfaces.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


