从热力学第二定律的角度对正十二烷/PODE3 混合喷雾的燃烧特性进行数值研究
IF 5.8
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
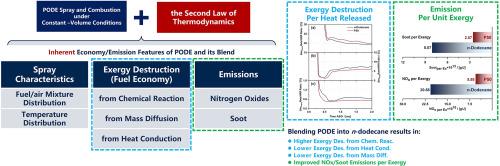
聚氧亚甲基二甲醚(PODE)作为一种潜在的电子燃料,可以实现内燃机的碳中和。现有关于 PODE 的研究主要基于发动机,由于发动机规格和运行条件不同,导致关于燃料消耗和污染物排放的结论相互矛盾。本研究在恒定容积条件下应用热力学第二定律分析燃油经济性和排放特性,不受特定测试条件或发动机类型的影响。对纯正十二烷和 PODE3/ 正十二烷混合燃料的基本燃料经济性和排放相关行为进行了数值研究。在喷射和燃烧过程中,与正十二烷相比,混合燃料在燃料/空气混合物较稀且温度较高的区域表现出低温放热(LTHR)。然而,混合燃料的高温放热(HTHR)更接近于化学计量燃烧,但温度更低。在正十二烷中掺入 PODE3 会增加化学反应引起的放能破坏,但会减少与热传导和质量传递有关的放能破坏,从而导致总体潜在最大燃油经济性基本不变。在低温和高当量比条件下,化学反应引起的放能破坏对温度和当量比更加敏感。对于不同的 PODE3/n-dodecane 混合物,这种敏感性趋势几乎是一致的。PODE3 的低温等温条件下化学反应引起的放能破坏高于正十二烷。将正十二烷和混合燃料的燃烧温度分别控制在 1760 K 和 1900 K 以上,可以减少化学反应引起的能量损失。此外,与正十二烷相比,混合燃料的氮氧化物(NOx)和烟尘都有所减少。值得注意的是,通过在正十二烷中掺入 PODE3,可以改善氮氧化物-化学能破坏以及氮氧化物-烟尘的权衡关系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Numerical study on the combustion characteristics of n-dodecane/PODE3 blend spray from the perspective of the second law of thermodynamics
Polyoxymethylene dimethyl ethers (PODE), as a potential e-fuel, can realize the carbon neutrality for internal combustion engines. Existing studies on PODE are primarily engine-based, leading to contradictory conclusions about fuel consumption and pollutant emissions due to the different engine specifications and operating conditions. This work applies the second law of thermodynamics under constant-volume conditions to analyze fuel economy and emissions characteristics without the influence of particular test conditions or engine types. The fundamental fuel economic and emission-related behaviors of pure n-dodecane and PODE3/n-dodecane blended fuels were numerically studied. For the spray and combustion processes, compared with n-dodecane, the blended fuel exhibits the low-temperature heat release (LTHR) in the region with leaner fuel/air mixture and higher temperature. However, the high-temperature heat release (HTHR) of the blended fuel is closer to the stoichiometric combustion but with lower temperatures. Blending PODE3 into n-dodecane increases the exergy destruction induced by chemical reactions but decreases the exergy destruction related to heat conduction and mass transfer, resulting in a basically unchanged overall potential maximum fuel economy. Heightened sensitivity of the exergy destruction from chemical reactions to temperature and equivalence ratio is found under low-temperature and high-equivalence ratio conditions. This sensitivity trend is nearly consistent for different PODE3/n-dodecane blends. The exergy destruction arising from chemical reactions for the LTHR of PODE3 is higher than that of n-dodecane. Less exergy destruction induced from chemical reactions can be achieved by controlling the combustion temperature higher than 1760 K and 1900 K respectively for n-dodecane and the blended fuel. Moreover, both nitrogen oxide (NOx) and soot are reduced for the blended fuel compared with n-dodecane. Notably, the trade-off relationships of NOx-chemical exergy destruction as well as NOx-soot, can be improved by blending PODE3 into n-dodecane.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Combustion and Flame
工程技术-工程:化工
CiteScore
9.50
自引率
20.50%
发文量
631
审稿时长
3.8 months
期刊介绍:
The mission of the journal is to publish high quality work from experimental, theoretical, and computational investigations on the fundamentals of combustion phenomena and closely allied matters. While submissions in all pertinent areas are welcomed, past and recent focus of the journal has been on:
Development and validation of reaction kinetics, reduction of reaction mechanisms and modeling of combustion systems, including:
Conventional, alternative and surrogate fuels;
Pollutants;
Particulate and aerosol formation and abatement;
Heterogeneous processes.
Experimental, theoretical, and computational studies of laminar and turbulent combustion phenomena, including:
Premixed and non-premixed flames;
Ignition and extinction phenomena;
Flame propagation;
Flame structure;
Instabilities and swirl;
Flame spread;
Multi-phase reactants.
Advances in diagnostic and computational methods in combustion, including:
Measurement and simulation of scalar and vector properties;
Novel techniques;
State-of-the art applications.
Fundamental investigations of combustion technologies and systems, including:
Internal combustion engines;
Gas turbines;
Small- and large-scale stationary combustion and power generation;
Catalytic combustion;
Combustion synthesis;
Combustion under extreme conditions;
New concepts.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


