OGT 介导的 HDAC5 O-GlcNAcylation 通过调节表观遗传修饰和蛋白水解的平衡促进骨生成
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
背景O-GlcNAc转移酶(OGT)负责将O-连接的N-乙酰葡糖胺(O-GlcNAc)连接到蛋白质上,调控从转录和翻译到信号传导和新陈代谢等多种细胞过程。本研究主要探讨 OGT 在成骨过程中的作用和机制。材料与方法 我们通过生物信息学分析发现,OGT在骨质疏松症中被下调,通过使用OGT抑制剂(或OGA抑制剂)以及条件性基因敲除OGT小鼠在体外和体内实验确定其在成骨分化中的作用,并通过定量蛋白质组分析和RNA-seq、qRT-PCR、Western印迹、免疫荧光、H&E、ALP、ARS、Masson染色、IHC、显微CT等方法探讨其具体机制。结果表明,OGT 对体外成骨和成骨细胞分化以及体内卵巢切除(OVX)小鼠均有积极影响。一致的是,有条件地缺失 OGT 的小鼠表现出骨量减少,而 O-GlcNAcylation 增强剂可部分恢复卵巢切除(OVX)小鼠的骨量。从机理上讲,定量蛋白质组分析和高通量RNAseq进一步揭示了HDAC5是内源性O-GlcNAcylation底物之一,HDAC5在Thr934上的O-GlcNAcylation可促进其转运至溶酶体并随后降解,因此,提高HDAC5的O-GlcNAcylation水平可导致其胞质裂解,从而减少其核进入并增强DNA转录。OGT介导的HDAC5的O-GlcNAcylation调节了其胞质蛋白水解和核进入之间的平衡,从而影响了Notch信号通路和DNA表观遗传修饰,进而在成骨过程中发挥作用。此外,该研究还强调了 HDAC5 O-GlcNAcylation 在控制表观遗传学中的关键功能。本文的转化潜力一方面,OGT 有可能成为未来临床诊断骨质疏松症(OP)的新生物标志物。另一方面,OGT糖基化底物HDAC5的小分子抑制剂或OGT激动剂(如水飞蓟素)都有可能成为未来预防或治疗骨质疏松症的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
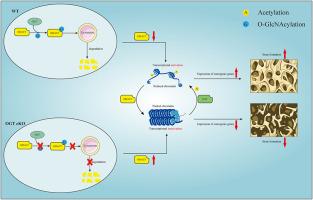
OGT mediated HDAC5 O-GlcNAcylation promotes osteogenesis by regulating the homeostasis of epigenetic modifications and proteolysis
Background
O-GlcNAc transferase (OGT) is responsible for attaching O-linked N-acetylglucosamine (O-GlcNAc) to proteins, regulating diverse cellular processes ranging from transcription and translation to signaling and metabolism. This study focuses on the role and mechanisms of OGT in osteogenesis.
Materials and methods
We found that OGT is downregulated in osteoporosis by bioinformatics analysis, determined its role in osteogenic differentiation by using OGT inhibitors (or OGA inhibitors) as well as conditional knockout OGT mice in vitro and in vivo, and explored and specific mechanisms by quantitative proteomic analysis and RNA-seq, qRT-PCR, western blotting, immunofluorescence, H&E, ALP, ARS, Masson staining, IHC, micro CT, etc.
Results
we revealed that OGT positively influenced osteogenesis and osteoblast differentiation in vitro as well as ovariectomy (OVX) mice in vivo. Consistently, mice with conditionally depleted OGT exhibited a reduction in bone mass, while O-GlcNAcylation enhancer could partially recover bone mass in ovariectomy (OVX) mice. Mechanistically, quantitative proteomic analysis and high-throughput RNAseq further reveals that HDAC5 is one of the endogenous O-GlcNAcylation substrates, and O-GlcNAcylation of HDAC5 on Thr934 promotes its translocation to lysosomes and subsequent degradation, thus, elevating the O-GlcNAcylation level of HDAC5 leads to its cytoplasmic cleavage, consequently diminished its nuclear entry and enhanced DNA transcription. The OGT-mediated O-GlcNAcylation of HDAC5 modulates the balance between its cytoplasmic proteolysis and nuclear entry, thereby impacting the Notch signaling pathway and DNA epigenetic modifications then playing a role in osteogenesis.
Conclusion
OGT is a regulator that promotes osteoblast differentiation and bone regeneration. Additionally, it highlights the critical function of HDAC5 O-GlcNAcylation in controlling epigenetics. This study offers fresh perspectives on osteogenesis and O-GlcNAcylation, proposing that the OGT-mediated O-GlcNAcylation of HDAC5 could be a promising target for osteoporosis treatment.
The translational potential of this article
On one side, OGT might potentially be used as a new biomarker for clinical diagnosis of osteoporosis (OP) in the future. On the other side, small molecule inhibitors of HDAC5, a glycosylation substrate of OGT, or OGT agonists such as silymarin, could all potentially serve as therapeutic targets for the prevention or treatment of OP in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


