通过 sp 或 sp2 杂化原子结合配体的 4、5 和 6 坐标镁配合物
IF 2.6
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
作为一种天然丰度很高的金属元素,镁在化学计量和最近的催化应用中都有广泛的用途。这通常利用了镁化合物的碱性或亲核性,或与其他有机金属化合物共络合的能力。然而,Mg(II) 的同色化学性质在很大程度上偏向于通过 sp2 和 sp3 杂化原子结合的烷基和芳基配体。在这里,我们将核磁共振光谱和 X 射线晶体学研究结合起来,对使用通过 sp(炔基)和 sp2(亚氨基)原子结合的配体的同性镁络合物进行更为罕见的替代 THF 溶剂研究。具体来说,我们利用末端炔烃和二苯基乙腈的高酸性,从商用烷基试剂 MgBu2 开始,制备了四溶解变形八面体配合物 Mg(C≡CC6H4R-p)2(THF)4 (R = Me,CF3)和 Mg(N=C=CPh2)2(THF)4 。使用二苯甲酮亚胺 Ph2C=NH 采用类似的去质子策略,可得到具有扭曲四面体镁中心的异极三核复合物 Mg3(N=CPh2)4nBu2(THF)2。使用格氏试剂对亚胺进行去质子化,或使用格氏试剂对腈进行亲核加成,都可以得到相关的亚胺卤化镁络合物,但这些不寻常的五配位络合物对 1,4-二氧六环诱导的施伦克平衡移动反应迟钝。采用较高的回流温度,从四氢呋喃介质转换到甲苯介质,可以获得同色亚胺配合物的四氢呋喃溶液,该配合物在 Mg3(N=CPh2)6(THF)2 中也具有三核结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
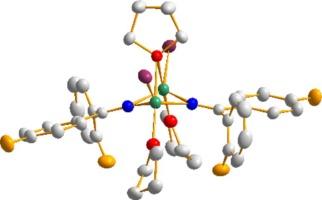
Magnesium 4, 5, and 6 coordinate complexes with ligands bound via sp or sp2 hybridized atoms
As a metallic element of high natural abundance, magnesium finds a wide range of uses in both stoichiometric and more recently catalytic applications. This often takes advantage of the basic or nucleophilic properties of its compounds or their ability to co-complex with other organometallic compounds. However, the homoleptic chemistry of Mg(II) is heavily skewed towards alkyl and aryl ligands bound via sp2 and sp3 hybridized atoms. Here, we report our combined NMR spectroscopic and X-ray crystallographic study into much rarer alternative THF solvates of homoleptic magnesium complexes using ligands which bind via sp (alkynyl) and sp2 (imido) atoms. Specifically, we exploit the high acidity of terminal alkynes and diphenylacetonitrile to prepare tetra-solvated distorted octahedral complexes Mg(C≡CC6H4R-p)2(THF)4 (R = Me, CF3) and Mg(N=C=CPh2)2(THF)4 starting from the commercial alkyl reagent MgBu2. Adopting a similar deprotonative strategy using benzophenoneimine Ph2C=NH affords the heteroleptic trinuclear complex Mg3(N=CPh2)4nBu2(THF)2 with distorted tetrahedral Mg centres. Related imidomagnesium halide complexes can be accessed either by deprotonation of the imine with a Grignard reagent, or nucleophilic addition of a Grignard reagent to a nitrile, but these unusual five-coordinate complexes are unresponsive to a 1,4-dioxane induced Schlenk equilibrium shift. Employing a higher reflux temperature switching from a THF to a toluene medium permits access to the THF solvate of a homoleptic imido complex which also possesses a trinuclear constitution in Mg3(N=CPh2)6(THF)2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


