2,5-噻吩二甲酸铀酰体系中的三周期框架:非识别辅助配体的影响
IF 2.6
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
以 N,N-二甲基乙酰胺(dma)为有机共溶剂,在溶液-水热条件下,2,5-噻吩二甲酸(H2tdc)与六水合硝酸铀酰发生反应,得到[UO2(tdc)(dma)](1)复合物,它与之前报道的[UO2(tdc)(nmp)](nmp = N-甲基-2-吡咯烷酮)同构。tdc2-采用双(μ2-κ1O:κ1O')桥接配位模式时,配合物 1 结晶为三周期框架,点符号为 {42.84}。以乙腈为有机共溶剂,在[Ni(PPh3)2Br2]的存在下,三苯基膦氧化物在原位形成,并与铀酰结合得到[UO2(tdc)(OPPh3)](2)。复合物 2 也是一个三周期框架,其点符号为 {4.102}2{42.104},拓扑类型为 dmd,铀为 3 配位 (3-c) 节点,tdc2- 在其双(μ2-κ1O:κ1O')桥接模式中为 4-c 节点,或在双(κ2O,O')螯合模式中为单边。在 1 和 2 中,与之前描述的 nmp 复合物一样,但不包括 [UO2(tdc)(dmf)](dmf = N,N-二甲基甲酰胺),未识别配体的配位破坏了最常见的与tdc2-和三螯合铀酰形成的二周期网络,并促进了框架的形成,在框架中,通道容纳了悬挂的未识别配体。络合物 2 在固态下的光量子产率为 3%,其发射光谱显示出典型的振子级数,峰位在具有 O5 赤道铀酰环境的络合物的常见范围内;室温下观察到的 "热带 "在 77 K 时消失。本文章由计算机程序翻译,如有差异,请以英文原文为准。
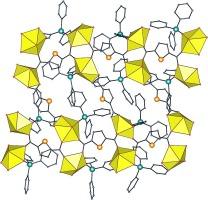
Triperiodic frameworks in the uranyl–2,5-thiophenedicarboxylate system: Effect of unidentate auxiliary ligands
2,5-Thiophenedicarboxylic acid (H2tdc) has been reacted with uranyl nitrate hexahydrate under solvo-hydrothermal conditions with N,N-dimethylacetamide (dma) as an organic cosolvent, giving the complex [UO2(tdc)(dma)] (1), isomorphous to the previously reported [UO2(tdc)(nmp)] (nmp = N-methyl-2-pyrrolidone). With tdc2− adopting the bis(μ2-κ1O:κ1O’)-bridging coordination mode, complex 1 crystallizes as a triperiodic framework with the point symbol {42.84}. With acetonitrile as an organic cosolvent and in the presence of [Ni(PPh3)2Br2], triphenylphosphine oxide is formed in situ and it binds to uranyl to give [UO2(tdc)(OPPh3)] (2). Complex 2 is also a triperiodic framework, with the point symbol {4.102}2{42.104} and the dmd topological type with uranium as 3-coordinated (3-c) nodes and tdc2− as either a 4-c node in its bis(μ2-κ1O:κ1O’)-bridging mode, or a simple edge in the bis(κ2O,O’)-chelating mode. In both 1 and 2, as in the previously described nmp complex, but not in [UO2(tdc)(dmf)] (dmf = N,N-dimethylformamide), coordination of a unidentate ligand disrupts the most common formation of diperiodic networks with tdc2− and tris-chelated uranyl, and promotes formation of frameworks in which channels accommodate the pendant, unidentate ligands. Complex 2 has a photoluminescence quantum yield of 3 % in the solid state, and its emission spectrum displays the typical vibronic progression with peak positions in the range usual for complexes with an O5 equatorial uranyl environment; the “hot band” observed at room temperature disappears at 77 K.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


