RAD18 催化形成的泛素化中间体模拟增殖细胞核抗原 PCNA
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
基于 2-((2-氯乙基)氨基)乙烷-1-硫醇(CAET)的化学诱捕策略是 E3 催化泛素化机理研究的实用工具。然而,通过 CAET 构建泛素化中间体模拟物(E2-Ub-底物共轭物)的方法仅限于肽,而其在折叠蛋白质底物上的应用仍有待探索。在这里,我们报告了在加入 PCNA 相关 E3 连接酶 RAD18 后,E2-Ub(RAD6A-Ub)和折叠蛋白底物 PCNA(增殖细胞核抗原)之间会形成二硫键。利用这一发现,我们采用内联蛋白拼接技术生成了一个稳定的、共价连接的 RAD18-RAD6A-Ub-PCNA 复合物,通过化学交联质谱(CX-MS)分析研究了这一复合物的结构。这项工作展示了利用底物相关的 E3 连接酶促进 E2-Ub 共轭物与折叠底物之间形成二硫键,以实现基于 CAET 的捕获,从而扩大了这项技术的应用范围。本文章由计算机程序翻译,如有差异,请以英文原文为准。
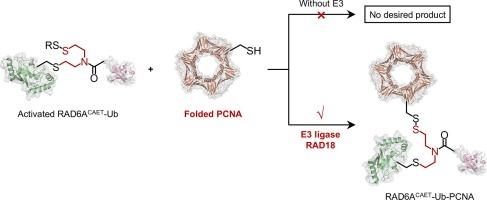
RAD18-catalysed formation of ubiquitination intermediate mimic of proliferating cell nuclear antigen PCNA
The 2-((2-chloroethyl)amino)ethane-1-thiol (CAET)-based chemical trapping strategy is a practical tool for mechanistic studies of E3-catalysed ubiquitination. However, the construction of ubiquitination intermediate mimics (E2-Ub-substrate conjugates) via CAET has been limited to peptides, while its application to folded protein substrates remains unexplored. Here, we report that disulfide bond formation between E2-Ub (RAD6A-Ub) and the folded protein substrate PCNA (proliferating cell nuclear antigen) occurs upon the addition of the PCNA-associated E3 ligase RAD18. Leveraging this finding, we employed intein splicing technology to generate a stable, covalently linked RAD18-RAD6A-Ub-PCNA complex, enabling chemical crosslinking mass spectrometry (CX–MS) analysis to study the structure of this complex. This work showcases use of a substrate-associated E3 ligase to promote disulfide bond formation between an E2-Ub conjugate and a folded substrate for CAET-based trapping, thereby expanding the scope of this technique.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


