抗体-药物共轭物革新癌症治疗的历程:综述
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
抗体药物共轭物(ADC)是一类强大的癌症靶向疗法,它利用单克隆抗体的特异性将细胞毒性有效载荷直接输送到肿瘤细胞,最大限度地减少了脱靶效应。本综述探讨了 ADC 技术的进展,重点是利用新型有效载荷、共轭策略和增强的药代动力学特征推进下一代 ADC 的发展。我们特别强调了创新的有效载荷,包括微管抑制剂、剪接体调节剂和 RNA 聚合酶抑制剂,它们提供了传统凋亡诱导之外的新细胞毒性机制。此外,尖端共轭技术的引入,如利用工程半胱氨酸进行位点特异性共轭、酶解方法和非天然氨基酸的整合,大大提高了 ADC 的均一性、有效性和安全性。此外,综述还深入探讨了 ADC 的作用机理,详细介绍了促进药物释放和细胞死亡的细胞内途径,并讨论了生物共轭方法在优化药物抗体比(DAR)方面的意义。此外,还探讨了建立 ADCdb 等综合数据库的问题,这些数据库收录了 ADC 的重要药理学和生物学数据,是推动 ADC 研究和临床应用的重要资源。最后,我们还探讨了 ADC 的临床前景,重点关注 FDA 批准的 ADC(如吉妥珠单抗-奥佐米星和曲妥珠单抗-恩坦辛)以及正在进行试验的新候选药物的发展。随着 ADC 的不断发展,其彻底改变癌症疗法的潜力依然巨大,为更有效、更个性化的治疗方案带来了新的希望。ADC 还融合了单克隆抗体的特异性和化疗药物的细胞毒性,在癌症靶向治疗方面取得了重大进展。因此,这种双重机制增强了肿瘤选择性,同时最大限度地降低了全身毒性,为更有效、更安全的癌症治疗铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
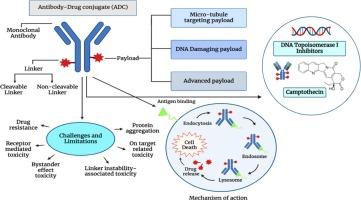
The journey of antibody-drug conjugates for revolutionizing cancer therapy: A review
Antibody-drug conjugates (ADCs) represent a powerful class of targeted cancer therapies that harness the specificity of monoclonal antibodies to deliver cytotoxic payloads directly to tumor cells, minimizing off-target effects. This review explores the advancements in ADC technologies, focusing on advancing next-generation ADCs with novel payloads, conjugation strategies, and enhanced pharmacokinetic profiles. In particular, we highlight innovative payloads, including microtubule inhibitors, spliceosome modulators, and RNA polymerase inhibitors, that offer new mechanisms of cytotoxicity beyond traditional apoptosis induction. Additionally, the introduction of sophisticated conjugation techniques, such as site-specific conjugation using engineered cysteines, enzymatic methods, and integration of non-natural amino acids, has greatly improved the homogeneity, efficacy, and safety of ADCs. Furthermore, the review delves into the mechanistic insights into ADC action, detailing the intracellular pathways that facilitate drug release and cell death, and discussing the significance of bioconjugation methods in optimizing drug-antibody ratios (DARs). The establishment of comprehensive databases like ADCdb, which catalog vital pharmacological and biological data for ADCs, is also explored as a critical resource for advancing ADC research and clinical application. Finally, the clinical landscape of ADCs is examined, with a focus on the evolution of FDA-approved ADCs, such as Gemtuzumab Ozogamicin and Trastuzumab Emtansine, as well as emerging candidates in ongoing trials. As ADCs continue to evolve, their potential to revolutionize cancer therapy remains immense, offering new hope for more effective and personalized treatment options. ADCs also offer a significant advancement in targeted cancer therapy by merging the specificity of monoclonal antibodies with cytotoxic potency of chemotherapeutic agents. Hence, this dual mechanism intensifies tumor selectivity while minimizing systemic toxicity, paving the way for more effective and safer cancer treatments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


