冷大气等离子体通过增强 KAT6A 乙酰化恢复三阴性乳腺癌中偏斜的巨噬细胞极化
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
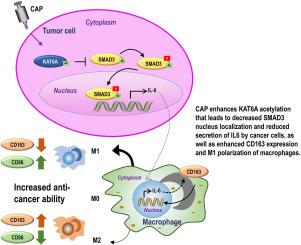
由于诊断和治疗方法选择不当,乳腺癌是世界上最常见的癌症,也是妇女癌症死亡的第二大原因。在治疗过程中,乳腺癌的分子特征可能会在亚型之间转换,从而导致表型,如更难治疗的三阴性乳腺癌(TNBC)。冷大气等离子体(CAP)在抑制多种癌症类型的恶性肿瘤方面的疗效已被多项研究证实,其中包括缺乏表面受体表达的 TNBC,因此它是乳腺癌中最难治疗的一种。通过分析一例被误诊为 BRCA1 突变的乳腺癌临床病例的基因检测报告,我们发现了 KAT6A 在推动该患者疾病进展中的重要性。通过探索在 KAT6A 和 SMAD3 之间的物理相互作用下受到不同调控的基因,我们提出了能够在乳腺癌免疫微环境中驱动巨噬细胞 M2 极化的 KAT6A/SMAD3/IL6/CD163 分子轴。通过研究 KAT6A 在转录和翻译水平上的表达图谱,我们提出了 KAT6A 乙酰化在降低其乙酰化 SMAD3 的能力以及随后的致癌作用中可能扮演的角色。通过分析 TNBC 细胞对 CAP 处理反应的整个转录组和乙酰组,我们预测了 CAP 通过增加 KAT6A 乙酰化对 TNBC 的疗效,并在体外和体内进行了验证。我们的研究首次提出了 CAP 在乳腺癌微环境中通过提高 KAT6A 乙酰化将巨噬细胞从 M2 状态重新极化为 M1 状态的作用,因此临床医生有必要仔细解读患者的基因检测报告,以减少因治疗方法选择不当而导致的死亡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cold atmospheric plasma restores skewed macrophage polarization in triple negative breast cancers via enhancing KAT6A acetylation
Breast cancer is the most common cancer diagnosed and the second leading cause of death of cancer among women in the world, due to inappropriate diagnosis and choice of therapeutic approach. The molecular profiles of breast cancers may switch among subtypes during treatments, leading to a phenotype such as triple negative breast cancers (TNBCs) that is more difficult to treat. Cold atmospheric plasma (CAP) has been demonstrated by many studies on its efficacy in arresting the malignancies of multiple cancer types including TNBCs that lack surface receptor expression and are thus the most difficult to treat among breast cancers. By analyzing the genetic testing reports of a breast cancer clinical case misdiagnosed with BRCA1 mutation, we characterized the importance of KAT6A in driving disease progression of this patient. Through exploring genes differentially regulated under physical interactions between KAT6A and SMAD3, we proposed the KAT6A/SMAD3/IL6/CD163 molecular axis capable of driving macrophage M2 polarization in the immune microenvironment of breast cancers. Through examining the expression landscapes of KAT6A at both transcriptional and translational levels, we proposed a possible role of KAT6A acetylation in reducing its ability in acetylating SMAD3 and subsequent oncogenic roles. Through analyzing the whole transcriptome and acetylome of TNBC cells in response to CAP treatment, we predicted the efficacy of CAP in resolving TNBCs via increasing KAT6A acetylation, which were validated both in vitro and in vivo. Our study, for the first time, presented the role of CAP in re-polarizing macrophages from the M2 to M1 state in the microenvironment of breast cancers via elevating KAT6A acetylation, and warranted careful interpretation of patients’ genetic testing reports by clinicians for the sake of minimizing mortalities due to inappropriate choice of therapeutic modalities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


