利用非谐波振荡器势计算研究磁场对双原子分子热函数的影响
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在本研究中,我研究了磁场对具有各向同性振荡器加反二次电动势的 H2、HCl 和 CO 等双原子分子热特性的影响。为此,我利用磁场存在时的势模型求解了薛定谔方程,并通过拉普拉斯变换方法获得了系统的束缚态。应用确定的能量特征值,我利用泊松求和公式计算了分割函数和热函数,如恒压下的比热、吉布斯自由能和存在外磁场时的焓。我将我们的结果与实验数据进行了比较,两者之间有很好的一致性。我计算了有磁场和无磁场条件下双原子分子的恒压比热、吉布斯自由能和焓的平均绝对偏差。所有计算出的平均偏差都小于 3%,这表明我们的计算是准确的。我的研究结果表明,外部磁场对双原子分子的热力学性质有重大影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
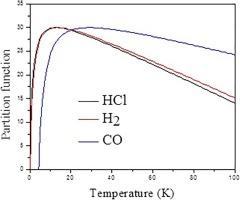
Computational investigation of magnetic field effect on thermal function of diatomic molecules with anharmonic oscillator potential
In the present study, I have investigated the magnetic field contributions on thermal properties of diatomic molecules such as H2, HCl and CO with isotropic oscillator plus inverse quadratic potential. To this end, I have solved the Schrödinger equation with the potential model in the presence of magnetic fields and have obtained bound states of the system via the Laplace transform approach. Applying the determined energy eigenvalues, I have computed the partition function and thermal function such as specific heat in constant pressure, Gibbs free energy and enthalpy in the presence of external magnetic field using Poisson summation formula. I have compared our results with experimental data and there is a good agreement between them. I have computed the average absolute deviations of specific heat in constant pressure, Gibbs free energy and enthalpy for diatomic molecules in the presence and absence of magnetic fields. All calculated average deviations are under 3 % that show our accuracy of computations. My results show that external magnetic fields have a significant influence on thermodynamic properties of diatomic molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


