发现新型环戊烷羧酸作为 NaV1.7 的强效选择性抑制剂。
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
环戊烷羧酸 31 是一种强效的 NaV1.7 抑制剂,对 NaV1.5 具有高选择性,并在遗传性红斑性肢痛症(IEM)转基因小鼠实验中表现出强大的镇痛效果,本文介绍了环戊烷羧酸 31 的发现过程。最终发现 31 的关键设计要素包括对脯氨酸取代基的探索、用环戊烷羧酸取代脯氨酸弹头,从而显著提高了 NaV1.7 的效力,以及用 2,6-二氯苄基取代的哌啶系统取代代谢易受影响的金刚烷基团,从而解决了代谢不稳定性问题,并显著改善了 PK。本文章由计算机程序翻译,如有差异,请以英文原文为准。
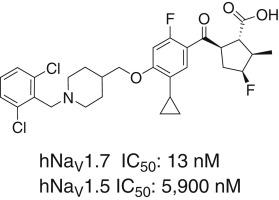
Discovery of novel cyclopentane carboxylic acids as potent and selective inhibitors of NaV1.7
Discovery efforts leading to the identification of cyclopentane carboxylic acid 31, a potent inhibitor of NaV1.7 that showed high selectivity over NaV1.5 and exhibited robust analgesic effects in an inherited erythromelalgia (IEM) transgenic mouse assay, are described herein. Key design elements that culminated in the discovery of 31 include exploration of proline substituents, replacement of the proline warhead with cyclopentane carboxylic acid, that led to significantly boosted NaV1.7 potency, and replacement of the metabolically labile adamantane motif with the 2,6-dichlorobenzyl substituted piperidine system, that addressed metabolic instability and led to a significant improvement in PK.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


