利用半乳糖氧化酶工程技术,从 d-葡萄糖†中高效级联合成 l-谷甾醇酸
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
L-Guluronic 酸是一种具有免疫调节和抗炎特性的专利药物,传统上是从天然海藻中提取或化学合成。这两种方法都存在选择性低和环境问题,因此人们亟需开发一种绿色高效的方法来生产 L-谷氨酰胺。在此,我们利用两种氧化酶--葡萄糖氧化酶(GOx)和半乳糖氧化酶(GOase)--成功开发了一条两步酶法路线,以 D-葡萄糖为原料合成 L-古醛酸。由于 GOase 在催化 D-葡萄糖酸氧化过程中的动力学反应迟钝,因此进行了合理的设计。通过分子对接和位点饱和突变,得到了 GOase V3(T406R/K330R/Y329F)变体。该变体对 D-葡萄糖酸的氧化活性显著提高了 13 倍,达到 7.8 U mg-1。这些取代协同将 D-葡萄糖酸分子转换为有利的构象,并促进了 C-6 位伯醇基团的特定活化。在级联反应中,40 mM D-葡萄糖在 2 小时内完全转化为 L-谷氨酰胺,选择性达到 100%,为可扩展的 L-谷氨酰胺生产提供了一种前景广阔的绿色生物制造策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
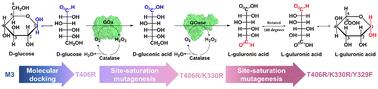
Engineering galactose oxidase for efficient cascade synthesis of l-guluronic acid from d-glucose†
l-Guluronic acid, a patented drug with immunomodulatory and anti-inflammatory properties, is traditionally sourced from natural seaweed species or synthesized chemically. Both methods suffer from low selectivity and environmental concerns and there is a high demand to develop a green and efficient approach for l-guluronic acid production. Herein, we successfully developed a two-step enzymatic route to synthesize l-guluronic acid from d-glucose using two oxidases, glucose oxidase (GOx) and galactose oxidase (GOase). Due to the sluggish kinetics of GOase in catalyzing the oxidation of d-gluconic acid, rational design was implemented. Through molecular docking and site saturation mutagenesis, a variant, GOase V3 (T406R/K330R/Y329F), was obtained. The variant exhibited a remarkable 13-fold higher oxidation activity towards d-gluconic acid, reaching 7.8 U mg−1. The substitutions synergistically switch the d-gluconic acid molecule to a favorable conformation and facilitate the specific activation of the primary alcohol group at the C-6 position. In a cascade reaction, the complete conversion of 40 mM d-glucose to l-guluronic acid was achieved in 2 h with 100% selectivity, presenting a promising and green bio-manufacturing strategy for the scalable production of l-guluronic acid.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


