Mo2C 上单原子 Cu 对 CO2/CO 加氢制甲醇的催化性能增强:第一原理研究†。
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
二氧化碳的排放会对环境造成危害,因为它在促进气候变化和海洋酸化方面起着关键作用。利用二氧化碳的一种方法是将其作为化学材料的前体,以实现能源转型。从绿色 H2 将 CO2 转化为甲醇是一个很有前景的选择。二氧化硅支撑的 Cu/Mo2CTx (MXene) 催化剂显示出比工业参考体系 Cu/ZnO/Al2O3 更高的活性。为了更好地理解 Cu/Mo2CTx 中的 CO2 加氢及反应条件下的相关过程(CO 加氢和反向水气变换反应),我们利用之前校准的理论模型和实验特征进行了周期性 DFT 计算,以评估甲醇合成反应机理。我们的结果表明,Cu/Mo2CTx 界面在提供低能途径以促进 CO2 加氢生成甲醇的过程中发挥了至关重要的作用,其中 Cu 原子和 Mo2CTx 支持物都参与了反应机理。研究结果展示了这种支撑型单原子催化剂所提供的独特途径,与基于氧化物上支撑的 Cu NPs 的经典异相催化剂相比,这种催化剂允许分子氢 (H2) 的连续异解裂解,形成 HCOO*、HCOOH* 和 H2COOH* 三种共吸附氢。因此,在反应条件下很容易形成 CH3OH。CO 也会通过反向水气变换 (RWGS) 反应形成,并可加氢生成甲醇。这些发现为利用单原子催化剂和金属支撑界面了解 CO2 和 CO 加氢开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
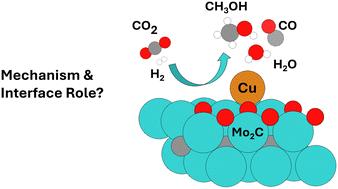
Enhanced catalytic performance of single-atom Cu on Mo2C toward CO2/CO hydrogenation to methanol: a first-principles study†
CO2 emissions harm the environment due to their pivotal role in fostering climate change and ocean acidification. One way to take advantage of CO2 is to use it as a precursor to chemical materials to enable energy transition. The CO2 to methanol conversion from green H2 is a promising option. The silica-supported Cu/Mo2CTx (MXene) catalyst displayed higher activity than the industrial reference system Cu/ZnO/Al2O3. To better understand CO2 hydrogenation in Cu/Mo2CTx and related processes under reaction conditions (CO hydrogenation and reverse water gas shift reaction), we performed periodic DFT calculations to evaluate the methanol synthesis reaction mechanism using our previously calibrated theoretical model against experiment characterization. Our results show the crucial role played by the Cu/Mo2CTx interface in providing low-energy pathways to facilitate the hydrogenation of CO2 to methanol, where both the Cu atom and the Mo2CTx support participate in the reaction mechanism. The findings showcase the unique pathways provided by this supported single-atom catalyst, allowing the successive heterolytic cleavages of molecular hydrogen (H2) to form HCOO*, HCOOH*, and H2COOH* species with co-adsorbed hydrogen in contrast with classical heterogeneous catalysts based on Cu NPs supported on oxides. Thus, CH3OH is readily formed under reaction conditions. CO also forms via the reverse water-gas shift (RWGS) reaction, which can be hydrogenated to methanol. These findings open new avenues to understanding CO2 and CO hydrogenation by exploiting single-atom catalysts and metal–support interfaces.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


