氢氧化物和氟化物修饰对二维 g-C3N4† 光催化活性的协同调控机制探索
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
剥离和杂质元素掺杂是 g-C3N4 带隙工程和增强光催化性能的常用程序。目前仍需要一种新颖、简便的策略来同时进行剥离和杂质元素掺杂。在此,我们使用不同浓度的乙醇和氢氟酸(HF),通过对块状 g-C3N4 (BCN)进行单步溶热剥离,制备了氢氧化物和氟化物掺杂的超薄 g-C3N4 纳米片。显微镜和光谱分析证实了氢氧基团和氟化物基团(HO-/F-基团)在 g-C3N4 纳米片(HF-CNS)中的掺杂。HO- 基团主要位于 HF-CNS 中庚嗪环的末端氨基上,而 F- 基团则可能是通过形成 C-F 键掺入 g-C3N4 晶格中。紫外-可见吸收光谱和 DFT 计算表明,电子带结构以及电荷载流子重组可在剥离过程中通过改变 HF 的量来调整。BET 比表面积从 BCN 的 18.65 m2 g-1 增加到 HF-CNS 的 159.87 m2 g-1。瞬态光电流从 5 μA 增加到 20 μA。HF-CNS 明显改善了四环素的光催化降解,在 50 分钟内达到 99% 的去除率,而 BCN 的去除率仅为 20%。四环素降解遵循伪一阶动力学,表观速率常数 (K) 从 BCN 的 0.0028 min-1 增至 HF-CNS 的 0.0793 min-1,提高了 30 倍。HF-CNS 的光催化氢进化是 BCN 的 11 倍。HF-CNS 表现出显著的稳定性和可重复使用性,表明它有望成为绿色制氢和降解有机污染物的光催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
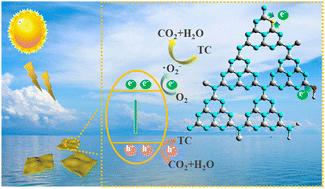
Exploration of the synergistic regulatory mechanism of hydroxide and fluoride modification on the photocatalytic activity of 2D g-C3N4†
Exfoliation and hetero-element doping are common procedures for band gap engineering and enhancing the photocatalytic properties of g-C3N4. A novel and facile strategy for simultaneous exfoliation and hetero-element doping is still in high demand. Herein, we prepared hydroxide and fluoride doped ultrathin g-C3N4 nanosheets through the single-step solvothermal exfoliation of bulk g-C3N4 (BCN) using varying concentrations of ethanol and hydrofluoric acid (HF). The microscopic and spectroscopic analysis confirmed the doping of hydroxide groups and fluoride groups (HO−/F− groups) into g-C3N4 nanosheets (HF-CNS). The HO− groups are primarily located at the terminal amino groups of the heptazine rings in HF-CNS, while the F− groups are likely incorporated into the g-C3N4 lattice by forming C–F bonds. The UV-vis absorption spectra and DFT calculations showed that the electronic band structure, and hence charge carrier recombination, can be tuned by varying the HF amount during the exfoliation process. BET-specific surface area was increased from 18.65 m2 g−1 for BCN to 159.87 m2 g−1 for HF-CNS. The transient photocurrent increased from 5 μA to 20 μA. HF-CNS significantly improved the photocatalytic degradation of tetracycline, achieving 99% removal in 50 minutes, compared to 20% for BCN. Tetracycline degradation followed pseudo-first-order kinetics, with apparent rate constants (K) increasing from 0.0028 min−1 for BCN to 0.0793 min−1 for HF-CNS, a 30-fold enhancement. The photocatalytic hydrogen evolution for HF-CNS was 11 times higher than that of BCN. The HF-CNS exhibited remarkable stability and reusability, indicating its potential as a promising photocatalyst for green hydrogen production and degradation of organic pollutants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


